



Control of Blackhead Disease
A thorough review of this hard-to-control disease of turkeys, chickens and other farmed birds was presented by Dr Larry R. McDougald of the University of Georgia at the 2008 North Carolina Turkey Days.Blackhead disease continues to cause sporadic but severe disease losses in commercial turkey flocks. Losses are more common but less severe in chickens, particularly broiler breeder pullets, and in game bird flocks reared in confinement and on open range. The wild turkey, a common game bird in North America, as well as peafowl and certain other gallinaceous birds often fall victim to this disease.
While death losses often reach 80- 100 per cent in domestic turkeys, a different face of the disease is seen in chickens. Broiler breeder pullets may suffer 10% mortality, extensive culling losses, and poor uniformity at time of lay. In the absence of any highly effective treatment drugs, emphasis on control is placed on prevention and containment by management and quarantine. Fortunately, research has been initiated in several laboratories in the USA and Europe, which are yielding important new findings which will be of value in planning future control programs. The older literature and some new findings were reviewed by McDougald (2005).
Additions to the life cycle of Histomonas meleagridis
At one time, researchers and diagnosticians believed that infections in turkey flocks arose from the ingestion of embryonated eggs of the caecal worm Heterakis gallinarum, or ingestion of earthworms that were carrying larvae of the caecal worm. This mechanism did not explain the phenomenon of rapid spread of blackhead through a flock of turkeys, and led some to question whether other intermediate hosts might be involved.
This question was addressed more recently in experiments where uninfected turkey poults were placed in pens alongside other directly inoculated poults, in the absence of any other possible carrier or host (McDougald and Hu, 2003). The results were clear-cut and dramatic. The uninoculated birds readily contracted the infection, became sick and died. By the end of the study, all of the birds had died or were sick with blackhead (Figure 1).
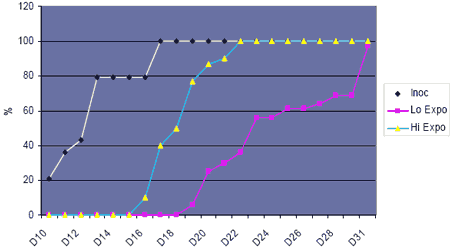
Further work on this means of acquisition of infection showed that the oral route was not involved, and that birds probably became infected by intake of liquid faeces in a process known as cloacal drinking (16).
The implication of this finding was that if healthy birds could be separated from sick birds, it would be impossible for the infection to spread through the flock. This could be accomplished by means of migration barriers, to divide the house into smaller sections, thereby limiting risk. This concept has been used successfully in some instances.
Where do turkeys get infections?
It is common that veterinarians and other investigators are unable to find Heterakis worms associated with outbreaks in turkeys. If the above discussion is considered, then it would be logical that the infection comes from outside the flock, probably tracked inside by a worker on the shoes. The source of such contamination is most likely chickens, which are often found not too distant from the turkey flock.
A review of the literature shows that chickens, among the domestic gallinaceous birds, are the best hosts for Heterakis worms, and that the eggs produced by these worms in chickens are the best for causing disease when inoculated into turkeys. Lund and Chute (1969) found that young chickens were 16 times as effective as mature chickens in hosting caecal worms, and that young turkeys were almost negligible in this respect. Lund and Chute (1973) tested eight species of gallinaceous birds and found that the Chinese ringneck pheasant was the best host for caecal worms, followed by chickens and guinea fowl.
In modern poultry production, it is not unusual for farms used for one type of poultry to be converted to rearing of another type. Probably the most disastrous example is the conversion of broiler breeder farms to the rearing of turkeys. It is commonly agreed that all broiler breeder farms are heavily contaminated with caecal worm (Heterakis gallinarum) eggs, which are the only known biological vector of the blackhead organism. (Earthworms can harbour caecal worms until they are eaten by chickens or turkeys, but this is only an 'extra' reservoir of infection and not a necessary part of the life cycle). Such farms reportedly remain infective to turkeys for many years.
Where did blackhead disease come from?
The first cases of blackhead in turkeys were reported from Rhode Island in 1892. It is interesting that the Chinese ringneck pheasant was introduced into the USA in 1881, and was soon propagated widely for release as a game bird. The chukar partridge has also been imported and propagated for release in many areas.
Blackhead outbreaks decimated the turkey industry in the New England area, and followed the farmers to the Midwest, Canada and to the far West. Up to World War II, blackhead was the leading cause of mortality in turkeys. Game birds are usually overlooked as a source of infection, but obviously could be another reservoir of infection as they may be present in areas where turkeys are raised. As shown by Lund, the pheasant and chukar are far better hosts for Heterakis than even chickens, and suffer little from the effects of histomoniasis, making them ideal as reservoirs of the disease.
How is blackhead controlled by management?
Prevention of blackhead in turkeys by management is two-fold:
- prevention of exposure by quarantine or isolation, especially avoiding any contact with chickens or game birds, and
- use of migration barriers to prevent commingling of infected birds with uninfected birds.
It is interesting that the contagious spread of blackhead by direct contact of birds is not as important in chickens as in turkeys (Hu et al. 2006). Thus, it is likely that infections in chickens result only from ingestion of Heterakis ova. Also, farm owners should be aware of the hobbies of their workers and discourage the keeping of backyard chickens, pheasants, chukars or fighting cocks.
Could a vaccination against histomoniasis be developed?
Previous investigators considered immunization an impractical approach to control of blackhead disease. Repeated infection and treatment with dimetridazole produced turkeys that were resistant to re-infection after three infection/treatment cycles.
Recent experiments in our laboratory were successful in demonstrating some protection when turkey poults were given two or more inoculations with an antigen consisting of freeze/thawed cultured H. meleagridis. A single inoculation failed to offer protection when birds were challenged within a few weeks.
More work is needed to identify the best vaccination regime, the amount of antigen needed, and the possible contribution of bacteria to the host immune response.
Does Histomonas respond to anticoccidials or antibiotics?
The answer to this is essentially no. Like some of its common relatives Trichomonas and Giardia, it is anaerobic and lacks mitochondria. These organisms make energy by an anaerobic process involving special organelles called hydrogenomes, This explains why histomonads do not respond to these other types of chemotherapeutic agents; they simply lack the metabolic machinery to be interfered with.
Sometimes birds infected with blackhead, particularly chickens, will seem to respond to antibiotic treatment. However, this is probably because of secondary infections with bacteria that could be affected by the drug.
Antibiotics normally have little beneficial effects on turkeys during a blackhead outbreak. Early and frequent preventive use of wormers (benzimidazole) can be of benefit in chickens because worms are the primary source of infection (6). In turkeys, where the infection spreads easily from bird to bird, this is probably not of value after outbreaks start.
Discussion and Conclusions
Even though losses from blackhead disease continue to be highly significant, some progress has been made in recommendations for control by management. Chickens and game birds are likely the most important source of infection for turkeys and other birds, as they are prolific in generation of infective cecal worm ova.
The life cycle of Histomonas has been reconsidered, after the discovery that turkeys could become infected from direct contact with other birds or contaminated faeces.
The unique structure and metabolism of Histomonas makes these organisms immune to treatment with anticoccidials and antibiotics, but antibiotics are usually considered beneficial to treat secondary bacterial infections.
References
1. Augustine, P.C. and Danforth, H.D. 1990. Invasion in foreign host birds and generation of partial immunity against coccidiosis. Avian Dis. 34:196-2022. Augustine P.C., Danforth, H.D. and Barta, J.R. 1991. Development of protective immunity against Eimeria tenella and E. acervulina in white leghorn chickens inoculated repeatedly with high doses of turkey Coccidia. Avian Dis. 35:535-541
3. Chute, M.B. and Chute, A.M. 1969. Freeze preservation of Histomonas meleagridis. Poultry Sci. 48:2189-2191
4. Fine, P.E.M. 1975. Quantitative studies on transmission of Parahistomonas wenrichi by ova of Heterakis gallinarum. Parasitol. 70:407-417
5. Graybill, H.W. and Smith, T. 1920. Production of fatal blackhead in turkeys by feeding embryonated eggs of Heterakis papillosa. J. Exp. Med. 31:647-655
6. Hegngi, F.N., Doperr, J., Cummings, T.S., Schwartz, R.D., Saunders, G., Zajac, A., Larsen, C.T., and Pierson, F.W. 1999. The effectiveness of benzimidazole derivatives for the treatment and prevention of histomonosis (blackhead) in turkeys. Vet. Parasitol. 81:29-37
7. Homer, B.L. and Butcher, G.D. 1991. Histomoniasis in leghorn pullets on a Florida farm. Avian Dis. 35:621-624
8. Horton-Smith, C. and Long, P.L. 1955. The infection of chickens (Gallus gallus) with suspensions of the blackhead organism Histomonas meleagridis. Vet. Rec. 67:478
9. Hu, J., Fuller, L. and McDougald, L.R. 2004. Infection of turkeys with Histomonas meleagridis by the cloacal drop method. Avian Dis. 48:746-750
10. Hu, J., Fuller, L. and McDougald, L.R. 2005. Blackhead disease in turkeys: Direct transmission of Histomonas meleagridis from bird to bird in a laboratory model. Avian Dis. 49: 328-331
11. Hu, J. and McDougald, L.R. 2002. Effect of anticoccidials and antibiotics on the control of blackhead disease in broiler breeder pullets. J. Appl. Poult. Res. 11:351-357
12. Hu, J. and McDougald, L.R. 2003. Direct lateral transmission of Histomonas meleagridis in turkeys. Avian Dis. 47:489-492
13. Lund, E.E. Oral transmission of histomoniasis in turkeys. 1956. Poult. Sci. 35:900-904
14. McDougald, L.R. 2003. Protozoal Infections. Chapter 29 in Diseases of Poultry, 11th ed. Edited by Saif, Y.M., Barnes, H.J., Glisson, J.R., Fadly, A.M., McDougald, L.R. and Swayne, D.E. Iowa State Press, Ames IA. 974-990
15. McDougald, L.R. and Hu, J. 2001. Blackhead disease (Histomonas meleagridis) aggravated in broiler chickens by concurrent infection with cecal coccidiosis (Eimeria tenella). Avian Dis. 45: 307-312
16. Sorvari, R., Naukkarinen, A. and Sorvari, T.E. 1977. Anal sucking-like movements in chicken and chick embryo followed by transportation of environmental material to bursa of Fabricius, ceca and cecal tonsils. Poultry Sci. 56:1426-1429.
January 2009
Further Reading
| - | You can view other presentations from the 2008 North Carolina Turkey Industry Days by clicking here. |








