
Few regions in the world are without IB virus
variants, he said. “In many countries, you have
three, four or five variants. We’re realizing that it
may be futile to vaccinate against one specific IB
virus strain. You have to try and make smart vaccine
combinations so your protection is broader.”
While IB variants can cause respiratory disease,
the greater concern in layers is a drop in egg
production and poor egg quality; in breeders, a
drop in hatchability might also occur. False layers
and nephritis are other possible consequences
of IB, De Wit said.
Low Titers = Poor Protection
Because they are long-lived birds, layers and
breeders need long-term IB protection. Live IB
vaccines can provide good protection but only for
a short period of time; they result in low antibody
titers, which correlate with low protection during
the laying period. Killed vaccines are required for
long-term protection but work much better if they
are administered after birds have received a live
vaccine first as a primer, he explained.
De Wit cited published, controlled studies
conducted by other investigators demonstrating
that layers not vaccinated against IB have the most
severe drop in egg production — above 70%.
A group vaccinated at 3 and 16 weeks of age with
a killed IB vaccine but no live primer had a drop
in egg production of about 30%, while a group
vaccinated with a live IB Massachusetts-strain
vaccine at 3 weeks of age then with a different live
IB Massachusetts-strain vaccine at 16 weeks of
age had about a 10% drop in egg production.
Even better results occurred in birds vaccinated
once with a live IB vaccine at 3 weeks of age,
followed by an inactivated IB vaccine at 15 weeks
of age. This group had no drop in egg production,
he said.
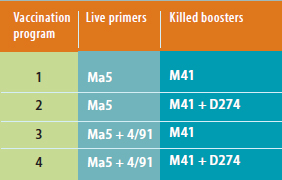
De Wit also presented the results of studies he
and colleagues conducted testing four IB vaccine
combinations against four Chinese Q1 IB strains
obtained from Latin America in 2009 and 2010. The
primer was either a live Massachusetts (Ma5) IB
strain or Ma5 plus a live IB 4/91 variant, which is
prevalent in Europe, followed by killed boosters
with IB M41 alone or with IB D274
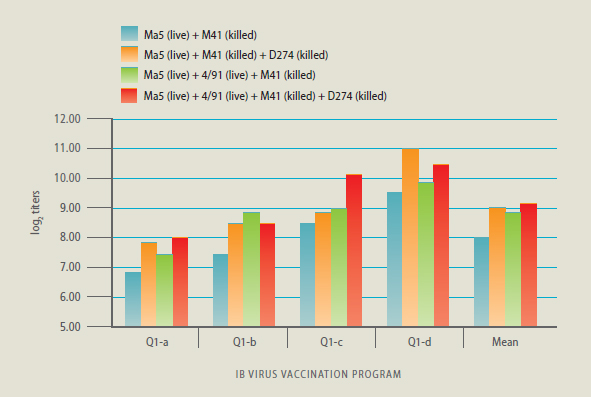
“The lowest level of virus-neutralizing antibodies
against this Q1 strain occurred when only a live
Massachusetts-strain (Ma5) vaccine and a boost
with inactivated M41 was used. On average, the
most complicated system of broad, live-vaccine
priming then a broad-boosting killed vaccine had
the best results and yielded the highest level of
neutralizing antibodies,” De Wit said.
Broad, Heterologous Bosting
“There are exceptions, but the message is usually
the same. It’s highly recommended, especially in
areas with a high IB challenge, that inactivated
vaccines be used to get more protection against
IB and, in general, that more vaccine strains be
used to achieve broad, heterologous boosting,”
De Wit said.
He also advised using a good, live, priming vaccine
before killed vaccines are administered to increase
the efficiency of the killed vaccines.
Even though good IB protection requires more
complicated vaccine programs, the good news,
he said, is that not every IB variant needs a vaccine
specifically for that variant. “By making smart
vaccine combinations, you can solve your
problems with a few vaccines.”
In addition, De Wit noted, “Producers quite often
complain that vaccination isn’t working because
there’s still a drop in egg production of about 5%,
but without vaccination, that drop could have
been 50%, 60%, 70% or even 80%.”
Vaccine Efficacy
Efficacy with IB vaccines, he pointed out, will
be affected by the strains of IB virus that are
administered, by the quality of each antigen per
dose and by the adjuvants used in each vaccine.
Proper vaccine application is imperative, De Wit
emphasized. In addition, trials conducted with
live IB vaccines have demonstrated that efficacy
improves if the ventilation system is turned off and
the lights are on. If the vaccine is administered in
water, there needs to be good water quality, low
in temperature and free of pathogens.
“Keep in mind that you are working with a very
sensitive virus that’s easy to kill. If you’re aware of
that, you’re on the path to better results,” he said.
Diagnostics
De Wit is a “big fan” of diagnostics when IB is
suspected. “Even if there are no problems, I think
it’s a good idea to obtain serological testing at the
end of each flock just to see if titers are high for IB,
because that means there was a challenge.”
It also means that the vaccination schedule was
working. The information can help producers
decide whether they should continue doing
what they’re doing, or that their program needs
adjusting, he said.
Certainly, he said, samples should be taken for
testing when there are clinical problems. “It’s very,
very easy to make incorrect conclusions if you don’t
obtain diagnostics. Maybe it’s not IB or it’s some
unexpected variant. If you don’t know that, plans
for the next flock will be wrong.”
Asked about the role of co-existing disease, De Wit
said that flocks with infections such as mycoplasma,
pneumovirus or other health problems are not
necessarily more susceptible to IB — but the
clinical signs after the IB infection will be more
severe and the recovery will be harder.
More Issues












