



Mechanisms of Action for Supplemental NSP and Phytase Enzymes in Poultry Diets
End-users should work closely with the specific enzyme products to obtain the optimal outcome desired based on the mechanism of action, recommended Craig L. Wyatt, Terri Parr and Mike Bedford of AB Vista Feed Ingredients Ltd in their paper to the Carolina Poultry Nutrition Conference 2008.Introduction
Recently, there has been an availability shortage of many key raw materials or limitations resulting in significant price increases thus more than ever, a great deal of pressure has been placed on nutritionists and feed manufacturers to maximise their efficiency of nutrient utilisation. In fact, ingredient costs have increased to such an extent that in many countries the final cost of feed has almost doubled.
In most feeds, the pressure has been on two nutrients in particular: energy and phosphorus. With limitations in the use of animal proteins in some markets and pressure from fertiliser use, phosphate prices have tripled in the past year. In addition, there has been a surge in the use of cereal grains and animal fats for biofuels significantly putting pressure on dietary energy costs. The net result is that the increase in shadow prices in least cost formulations (LCF) for energy and phosphorus has driven feed manufacturers to consider alternative options especially feed enzymes.
The use of enzymes for releasing more energy from corn-based diets has increased especially in poultry in most markets compared to the past when the economic incentive was not great enough to overcome the scepticism. Some nutritionists are also formulating with considerably higher phytase dosages than in the past. Not only does this latter activity increase phosphorus retrieval from phytate but it will spare some energy as a result because of the removal of more phytate.
Most of the enzymes were derived from fungal sources but today, the degree of bacterial-derived enzymes has increased especially with the new phytase products. In the past five years, the market for poultry enzymes has changed significantly with phytases now leading followed by xylanases and then cellulases (glucanases) trailing in a distant third place. Other enzyme classes such amylases, proteases and mannanases are making up only a small proportion of the total feed enzyme market today.
Although there are some enzymes that are positioned at targeting vegetable protein sources (such as alpha-galactosidases), their use to date is still small and the understanding of the mode of action remains limited.
The main use of feed enzymes still targets cereal grains and in countries where wheat and barley predominate, the dietary enzyme use is almost universal. More effort has been put in developing a better understanding of how enzymes can enhance corn or sorghum and soybean meal diets for poultry and today this area is now increasing with the new better enzymes.
One fact that must be appreciated is that exogenous enzymes (including phytases) function through enhancing the availability and retention of nutrients present in the diet. If the nutrients 'spared' such as phosphorus (P) and energy do not happen to be the nutrients limiting animal performance, then there will be a small or no response upon addition of the enzyme.
Since ingredients vary markedly in their nutritive value, it is quite conceivable that two separate diets of an identical formulation based on corn and soya but derived from different sources (locations) will respond differentially upon enzyme administration. Thus, one must consider feed enzymes including phytase as another tool to help reduce variation in performance.
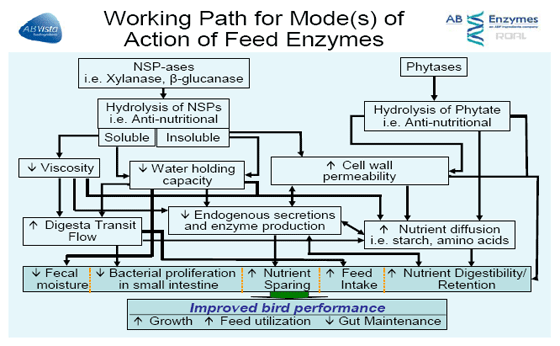
This paper will briefly discuss some of the possible mechanisms of action of feed enzymes shown in Figure 1 in diets fed to poultry and discuss some of the conditions which are known to influence the response observed.
PHYTASE
With the advent of commercial feed phytases, which is an enzyme class not constrained by cereal type, its use has become more commonplace to the point where today it constitutes more than half of all feed enzyme sales [<$250 million].
Phytases have been fed to poultry and pigs to degrade plant phytate that would otherwise pass through the digestive tract relatively intact with minimal release of P resulting in high levels of bound P in manure. Moreover, since phytate is in the form of calcium and magnesium salts, and is able to chelate many cations, its hydrolysis has been associated with improved mineral utilisation overall.
Consequently, most people feel feeding a phytase source can be effective for increasing P and mineral digestibility and retention, and as a result the use of inorganic P sources can be reduced.
In the past, phytase was simply used to provide phosphorus to the bird and as such, a small savings in feed costs was obtained by decreasing the use of inorganic phosphate sources in the diet.
Alternatively, new data derived from feeding second generation phytase sources have shown improvements beyond phosphorus with increases in retention of calcium, energy and amino acids.
The economics of such activities will differ markedly depending upon current prices of the nutrients spared but will result in a significant feed cost savings. In turn, this leads to the challenge that the nutritionist must know 'that all phytases work differently', leading to different performances in the animal.
Review of the early phytase data, based significantly on the fungal-derived phytases, suggest that there is a great deal of variability in the performance response when feeding these products (Rosen, 2002), including P digestibility (van der Klis et. al.,1997). This has lead to many nutritionists and feed formulators using a safety margin of lowering phosphorus and calcium levels in the nutrient matrix which significantly reduces the monetary value provided from feeding the phytase.
Mode of Action
Action in the acidic region of the gut
Many factors have been found to influence the response of the animal to these different phytases including the molecular properties themselves. Some commercial phytases have been found to have limited stability in the gastric environment and low specific activity compared to the new bacterial-derived phytases.
It has been clearly shown that phytate solubilises in the acidic region of the chicken gut, thus this is the critical area of digestion where one needs a highly efficacious phytase to work (Tamim, et al., 2004). In addition to the phytase working in the acidic environment, it needs to work quickly with a high affinity for the target substrate before being impacted by endogenous and exogenous proteases, i.e. pepsin.
While the low pH environment in the gastric phase is ideal for making phytate susceptible to hydrolysis by phytase, the low pH also places a stress on all proteins, including enzymes. For some fungal phytases, this appears to be a greater problem than for other phytases. The acidic phase (pH 2.5-4.5) of the intestinal tract is the only part where phytate is truly soluble and most likely susceptible to degradation. It is essential that a phytase maintains maximum activity across the pH range where phytate is most susceptible to hydrolysis (pH 4.5 and lower).
Several new phytases have been developed and marketed (Quantum™ Phytase and Finase EC) that clearly are better at working in the optimal acidic pH range compared to other phytase sources giving a more efficacious and consistent response.
Phytate as an anti-nutrient
As more phosphorus is removed from phytate, leading to more breakdown of intact IP-6, the less able it is to bind or chelate minerals, starch or proteins either directly or via ionic bridges (Selle & Ravindran, 2007). Decreasing the binding of these compounds through the use of phytase may directly improve the digestibility not only of phosphorus and divalent cations such as Ca, Zn and Mg, but also indirectly increase energy and nitrogen utilization.
Recently, it has been suggested that phytate alone is more of a potent antinutrient than previously thought, and as such its presence results in a significant loss of endogenous nutrients and energy in the form of mucins, intestinal cells and perhaps pancreatic enzymes (Cowieson et. al., 2004).
Exactly how it brings about such losses is probably multi-faceted but nevertheless, new data clearly indicates there are gains in bird performance from the hydrolysis of dietary phytate beyond that required to supply the phosphorus requirements of the bird from a typical phytase containing diet.
More recent work has demonstrated the influence of phytate on the digestibility of energy and amino acids (Wu et. al., 2003; Selle et. al., 2003; Selle et. al., 2006), either via direct interaction with positively charged amino acids or through deprivation of activating cations from digestive enzymes or as mentioned above excessive losses of mucins. This would clearly suggest that phytate is an intestinal irritant. It is not clear, however, how this effect is brought about, although recent evidence suggests that in addition to the decrease in absorption there is a reduction in inflammatory response measures at the duodenal/jejunal junction on feeding phytase to laying hens (Koutsos et al., 2005).
It is interesting that the effect of phytase on amino acid digestibility tends to be greatest on those amino acids that are prevalent in intestinal maintenance and turnover, namely cystine, threonine, proline and glycine when measured (Selle et. al., 2006). This suggests that a greater proportion of the benefit of phytase is due to reduced endogenous losses rather than increased dietary amino acid digestibility.
Thus, destruction of phytate reduces the anti-nutritive effect in a directly proportional manner and as a result, energy and amino acids that would have been used in a maintenance activity can be directed towards productive energy (growth) instead. The savings in endogenous losses can be directed towards growth, so that a greater proportion of metabolised energy and amino acids are used to net energy and less for maintenance.
It must be noted that this effect of phytase is mostly a post-adsorptive effect and if this is consistently the case, then it would explain why digestibility assays are not effective in determining the value in a phytase in sparing energy and amino acids for growth. Dietary phytate enhances and phytase reduces endogenous losses in what appears to be a dose-dependent response, thus feeding phytase appears to favour shifting ME to NE of growth rather then NE of maintenance. Low P diets will depress feed intake and it has been shown that usually more phytase is required to equilibrate intake than the available phosphorus (AvP) with the positive control. This can result in digestibility trials lacking the precision such that the calculated P equivalency exceeds that achieved in commercial practice.
Commercial Application
Unlike the NSP’ase enzymes used for corn soy diets, the phytase levels fed in practice are well below that of the biological optimum. Although the magnitude will vary between phytase sources the benefit has been found to be linearly related to logarithmic increments in dose – thus improvement in phosphorus digestibility is doubled with a ten-fold increment in dose. Despite new data supporting additional nutrient matrices beyond phosphorus and calcium for most phytases into energy and amino acids, the economic incentive to increase the dosage of phytase used has not been sufficiently obvious until recently with increasing ingredient costs.
A clear problem in the use of phytase in least cost formulation programmes (LCF) is that whilst the benefit of the enzyme increases in a linear fashion with logarithmic increments in dose, the LCF program does not account for the logarithmic benefit.
To demonstrate this issue, if a phytase is allowed to float as an ingredient with a fixed nutrient matrix for 500 units, and the LCF selects only 250 units of phytase, it will assume that the phytase has provided only 50 per cent of the given matrix, whereas in reality the actual value is closer to 75 per cent for such a dose. On the other hand, if the formulation programme doubles the inclusion level to 1000 units using the 500 unit matrix then one will significantly over-value the nutrient matrix supported by that product (potential loss in performance). Use of any dose below the product maximum results in the LCF assuming that the enzyme delivers less value than it actually does in vivo.
AB Vista has been looking at several different approaches to help formulators use the optimal amount of phytase. One solution is based on a dose model using shadow prices to best predict the optimal level of phytase for that situation. Another approach would be to place two phytase ingredients in the LCF programme based on one phytase having the recommended 500 unit nutrient matrix and the second would be a new phytase (500 to 1000 unit) product, which would have a matrix defined as the difference between the 500 and 1000 unit matrix products. Either approach is designed to help maximise the value that a feed manufacturer can extract from their phytase, especially when high ingredient prices justify much greater inclusion levels of this enzyme.
CARBOHYDRASE OR NSP ENZYMES FOR CORN-BASED DIETS
Recently, with dramatic increases in the price of dietary energy many nutritionists have renewed interest in feeding NSP enzymes due to the potential cost savings they offer. It has been a hard market to penetrate through customer acceptance because most end-users think that the best diet to feed poultry is a corn-soy diet, whereas diets based on barley or wheat are known to have negative issues like wet litter making the solution of feeding a specific enzyme more accepted with quick visible value, i.e. dry litter).
The challenge has been the inconsistent and/or muted responses in certain diets to feeding these types of enzymes primarily based on xylanases, glucanases, cellulases and mannanases. A significant body of work has shown these products do work but their responses can be quite variable because such a response in corn-soy diets requires the correct application adjustments.
Based on the industry's experience with NSP enzymes, the response can be variable depending on what enzyme or cocktail of enzymes used, the quality of feed ingredients or substrate and if the enzymes are thermo-tolerant. The economics of such activities will differ markedly depending upon current market prices of the nutrients spared but will result in significant feed cost savings. In turn this leads to the challenge that the nutritionist must know 'that all NSP enzymes work differently', leading to different performance in the animal.
Mode of Action
Enzymes targeting corn-soya diets were introduced many years ago to target several substrate components within the plant material including fibre, starch and some plant proteins.
It is thought that NSP enzymes function through a composite of three separate activities, the contribution of each activity varying with ingredients and individual birds. The two main key functions for a corn-soya based diet would be plant (cereal) cell wall destruction, and stimulation of beneficial bacteria with changes in the fiber composition.
The target for such a feed enzyme(s) product for corn-soya diets is not based on lowering intestinal viscosity derived from soluble cell wall polysaccharides since corn and soya contain low levels of soluble material. Instead of soluble fibre, it has been suggested that in corn-based diets, the target is the insoluble fibre component to help break open the cell wall material.
Research has shown that in addition to the impact of soluble fibre, the insoluble fraction can have a direct and indirect effect on digestive function. Several authors have shown that insoluble fibre, i.e. cellulose, has an inhibitory effect in vitro on lipase and proteases from the pancreas, and this has also been found in vivo trials (Almirall et al., 1995; Schneeman et al., 1985). The feeding of varying levels of grass and other NSP levels has been shown to increase the mass of the intestines in chickens. These changes in the digestive tract will lower digestive efficiency and increase the level of nutrients used for gut maintenance, thus pulling nutrients away from growth.
Whether the target is the soluble or insoluble component, the benefit brought about is due to enhanced digestibility and absorption, not only of the nutrients present in grain (like starch) but also from other ingredients, particularly added fats by improving the diffusion of nutrients within the intestinal lumen. The key functions described focus on how certain NSP enzymes function.
Cell wall destruction: encapsulation of nutrients
The cell wall material in the starchy endosperm of corn and sorghum is constructed mainly of small amounts of cellulose encrusted with hemicellulose, the bulk of which is arabinoxylan with minor beta-glucan components and lesser contents of mannans (Stone, 2004). Since poultry do not possess the necessary enzymatic capacity to degrade plant cell walls, a lot of the content (especially starch and protein) within this material can effectively bypass digestion or not be broken down until the lower gut by bacteria. This factor of encapsulation is based on the fact that some endosperm cells in corn and other ingredients manage to avoid physical breakdown during the activities of grinding and pelleting in feed manufacturing, and gizzard activity.
Effective degradation of this material requires the addition of sufficient amounts of the appropriate NSP enzyme activity such that 'holes' are created in the cell wall. This allows water hydration and large enough amounts of pancreatic proteases and amylases enabling better digestion of the starch and protein more rapidly. Xylanases, and to a lesser extent cellulases (beta-1,4-glucanases) have proven most effective in the field (Zanella et al., 2004; Leslie et al., 2007). Mannanases and pectinases have targeted the soy more so than the corn fraction of the diet, but with the same endpoint in mind (Jackson et al., 2004).
Many studies have shown improvements in starch and to a lesser extent, protein digestibility, which is indicative of activity of the enzyme towards corn endosperm cell walls. It has been shown that corn starch digestibility in the upper part of the digestive tract can vary markedly across different corn sources probably related to differences in starch structure, protein matrix, and the handling of the corn post-harvest (drying and milling/processing techniques). These products focus on breaking down the cell wall material exposing more starch and protein for enzymatic digestion, reducing endogenous secretion, i.e. lowers mucin production, and altering the lower gut microbial populations. These effects of exposing more nutrients in the upper intestine, reducing the physical damage and lowering endogenous secretions by the gut villa does lower maintenance requirement spent on digestion and improve nutrient retention.
However, in a corn-soy based diet, one will need to use NSP enzymes, i.e. xylanase, glucanase, that are more effective at targeting and breaking down the insoluble fibre fraction. A direct benefit of feeding these enzyme products is through reducing the variability in birds and improvements in bird uniformity across the different feed batches.
Bacterial population stimulation
Exogenous NSPases break down plant cell wall carbohydrates and reduce chain length producing smaller polymers and oligomers. At some point, the fragments become small enough, i.e. short-chain oligosaccharides, and numerous enough to act as a substrate (pre-biotic) for bacterial fermentation.
Xylanases, mannanases and cellulases produce xylo-, manno- or gluco- oligosaccharides, respectively. The benefit of such end products depends upon the type and quantity of the oligosaccharides produced, with different enzymes producing different oligosaccharides. These short chain oligosaccharides travel to the lower gut and become substrates for bacterial fermentation in the ileum and caecum, which can be beneficial with VFA production and altering the bacterial population.
Several papers have shown that use of enzymes significantly alters VFA production and the population profiles of bacteria in both the ileum and caecum (Apajalahti et. al., 1995; Choct et al., 1999; Apajalahti and Bedford 1999; Bedford & Apajalahti, 2001). Results have clearly shown the presence of medium- to long-chain oligomers will increase fermentation in the jejunum and ileum region. However, feeding a xylanase significantly decreases fermentation and shifts it to the caecum.
If the diet is well digested in the upper part of the digestive tract by using NSP’ase, it appears that there is little starch present in the ileum, and more cell wall oligomers present in the ileum and cecum (Engberg et. al., 2004). However, one must be careful in selecting the feed enzyme because some products can be overdosed and reduce the size of the oligosaccharides down to far to monosaccharides. If sufficient monosaccharides are produced, it may result in osmotic diarrhoea and /or poor performance (Schutte, 1990). These problems are most likely to occur with endo-xylanases products derived from a crude preparation containing substantial amounts of exo- rather endo-xylanase activity, which are not too specific in their requirements for binding to substrate.
As we learn more about these enzymes and their actions it is critical to obtain the right amount of short chain oligosaccharides, i.e. tri and di-saccharides, otherwise this alteration in the bacterial population in the lower gut may lead to increasing the gut maintenance requirements of the bird especially without growth promoters resulting in fewer nutrients for growth instead of a positive benefit.
Commercial Application
There are many different NSP enzymes on the market today, all of which differ markedly from one another. Even within the xylanase class, there are enormous differences in pH profiles, gastric stability, end products produced and their ability to attach to soluble and/or insoluble arabinoxylan structures.
It is important that when making a choice the decision is based on the animal performance of the product in proper formulated diets and not simply based on in vitro assays or TME/AME digestibility studies that can truly mislead the true net energy response.
The translation of such net energy response into a performance benefit has been very difficult to demonstrate because today's broilers do not respond to overfeeding energy. Nevertheless there have been studies demonstrating that not only ileal energy digestible levels of corn/soy diets can vary but also that inclusion of an appropriate enzyme can reduce this variation having a significant impact on body weight uniformity.
The value for the reduction in performance variability will be different across end-users and will need to be evaluated individually. Recent work would suggest if the proper amounts of amino acids are present the bird will respond positively to the increase in energy release especially in the heavy strain birds.
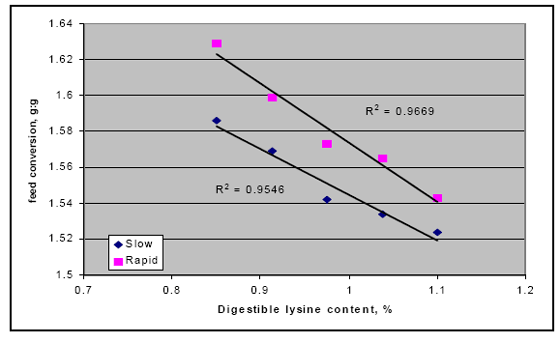
There also may be an interaction between rapid starch digestion and the need for more lysine. Several trials have shown that increasing the release of starch in the upper part of the digestive tract by feeding different starch sources or using enzymes to shift starch availability appears to respond better in higher lysine diets (Figure 2; Weurding et. al., 2002). These results indicate that nutritionists may need to adjust the diets to account for this release in nutrients to obtain optimal performance from products like enzymes.
PHYTASE AND CORN/SOYA NSP ENZYME COMBINATION APPLICATION
The combined use of phytase and NSP enzymes has become a critical subject of interest with many questions focusing on application. While we have a significant understanding of the use of phytase in general fed alone in poultry diets, the database for the combined use of phytase with different NSPases is quite limited. Nevertheless, knowing that phytases (especially Quantum) and NSPases target different substrates releasing nutrients like phosphorus and starch, and are effective in different regions of the gastro-intestinal tract, their indirect benefits of reducing gut maintenance (i.e. lower mucin production) may overlap. Thus, one might deduce that the combined effect may be complimentary but not directly additive.
Independently published research has shown that the interaction of feeding these products together based on mineral retention improves the responses giving better results than feeding either product alone. However, the data clearly showed there was no additive benefit on overall animal performance. In fact, the ability of the NSPase to physically break open the fibre components of the diet may improve the access of phytase to phytate increasing its hydrolysis and the action of both enzymes could be complementary, however there was no additional increase in net energy.
Thus, not knowing for certain the level of energy and amino acids resulting from the feeding of NSPase in corn-soya based diets; many people use a safety margin with these products. Current recommendation would be when feeding the proper NSPase product and phytase, i.e. Quantum, together, the net energy response between the two should be complementary in the bird. Based on feeding trials with phytase and NSPase, it is suggested that the combined use of these enzyme products may only result in partial additivity for energy thus approximately 80 per cent of the total. For instance, one kilocalorie ME of phytase and one kilocalorie ME of an NSP enzyme, when fed together, may only result in 1.6 kcals ME as opposed to an additive effect of 2 kcals.
Conclusions
There are many feed enzyme products available for use in poultry diets containing corn, wheat, soybean meal and other ingredients. The enzyme efficacy can vary substantially depending upon the type, e.g. phytase or xylanase, and source of the products. Also, the response can vary depending if the product is thermo-tolerant and able to survive the normal pelleting process (Parr and Wyatt, 2006). The intrinsically thermo-tolerant enzymes are clearly the best solution, with compromises arising from the use of either coating or post-pelleting application which vary with each product. In addition to surviving feed processing, the enzyme needs to be able to work in the right areas of the digestive tract in a highly efficacious way to result in an improvement in nutrient digestion and retention. Thus, it is important for the end-user to remember that not all enzymes within a class, e.g. phytase and xylanase, work the same resulting in different animal responses and different total values.
Typically, the response to phytase is more predictable across all diets compared to a xylanase product used specifically for corn-soy containing diets. This is a result of the fact that the target substrate is better defined for a phytase, and consequently resulting in a more consistent desired effect. Corn, soybean meal and other plant-based ingredients lack clear 'targets' until now. We have a better understanding that one needs to break down the insoluble cell wall material, which will result in a consistent nutrient increase compared to enzymes targeting the soluble fraction. Digestibility experiments do not provide a clear picture of the potential value of an enzyme product under commercial conditions because the response is coming from net energy, i.e. gut maintenance. There are dietary, feeding processing and/or environmental factors that can limit or extend the value of these products.
The end-user is encouraged to work closely with the specific products to obtain the optimal outcome desired based on the mechanism of action.
References
Almirall, M., Francesch M., Perez-Vendrell A.M., Brufau J. and Esteve-Garcia E.. (1995) The differences in intestinal viscosity produced by barley and β-glucanases alter digesta enzymes activities and ileal nutrient digestibilities more in broilers than in cocks. J. Nutr. 125: 947-955.
Apajalahti, J. & Bedford, M.R. (1999) Improve bird performance by feeding its microflora. World Poultry, 15, 20.
Apajalahti, J., Morgan, A.J., Lauraeus, M. & Heikkinen, P. (1995) Feed enzymes indirectly modify the microbial community of the gastrointestinal tract of broilers. BSAS Winter meeting, Scarborough.
Bedford M.R. & Apajalahti J. (2001) Implications of Diet and Enzyme Supplementation on the Microflora of the Intestinal Tract. In ADVANCES IN NUTRITIONAL TECHNOLOGY Proceedings of the 1st World Feed conference, pp. 197-206 [AFB van der Poel, JL Vahl, and RP kwakkel, editors]. Utrecht: Wageningen Pers.
Choct M., Hughes R.J. & Bedford M.R. (1999) Effects of a xylanase on individual bird variation, starch digestion throughout the intestine, and ileal and caecal volatile fatty acid production in chickens fed wheat. Br 40, 419-422.
Cowieson, A.J., Acamovic, T. and Bedford, M.R. (2004) The effects of phytase and phytic acid on the loss of endogenous amino acids and minerals from broiler chickens. British Poultry Science 45[1], 101.
Engberg, R.M., Hedemann, M.S., Steenfeldt, S. & Jensen, B.B. (2004) Influence of whole wheat and xylanase on broiler performance and microbial composition and activity in the digestive tract. Poultry Science, 83, 925.
Jackson M.E., Geronian K., Knox A., McNab J. & McCartney E. (2004) A dose-response study with the feed enzyme beta-mannanase in broilers provided with corn-soybean meal based diets in the absence of antibiotic growth promoters. Poult Sci 83, 1992-1996.
Koutos, E., Wyatt, C.L. and Bass P.D. (2005) Effect of dietary phytase level and source on second cycle commercial laying hens. I. Effects on hen body weight, carcass composition and intestinal histology. Poultry Science 84[Suppl. 1]: 107.
Leslie M.A., Moran E.T. Jr. & Bedford M.R. (2007) The effect of phytase and glucanase on the ileal digestible energy of corn and soybean meal fed to broilers. Poult Sci 86, 2350- 2357.
Parr, T. and Wyatt, C. (2006) Effect of different phytase sources on broiler performance and yield when added to the mixer and pelleted at two temperatures. Poultry Science 85[Suppl. 1]: 90.
Rosen, G.D. (2002) Microbial Phytase in broiler nutrition, 1st edn, p. 105. Nottingham University Press, Nottingham.
Schneeman, B.O. and Gallaher D. (1985) Effects of dietary fiber on digestive enzyme activity and bile acids in the small intestine. Proc. Soc. Exp. Biol. Med. 180:409-414.
Selle, P.H. and Ravindran, V. (2007) Microbial phytase in poultry nutrition. Animal Feed Science and Technology, 135:1 (Review).
Selle, P.H., Bryden, W.L., Pittolo, P.H., Ravindran, G. & Ravindran, V. (2003) Influence of phytase and xylanase supplementation on growth performance and nutrient utilisation of broilers offered wheat-based diets. Asian-Australasian Journal of Animal Sciences, 16, 394-402.
Selle, P.H., Ravindran, V., Bryden, W.L. and Scott, T.A. (2006) Influence of dietary phytate and exogenous phytase on amino acid digestibility in Poultry: A review. J. Poul. Sci. 43: 89.
Schutte J.B. (1990) Nutritional implications and metabolizable energy value of D- Xylose and L-Arabinose in chicks. Poult Sci 69, 1724-1730.
Stone B.A. (2004) Cell walls of cereal grains. pp. 1-9. Minneapolis: AACC.
Tamim, N.M., Angel, R. and Christman, M. (2004) Influence of dietary calcium and phytase on phytate phosphorus hydrolysis in broiler chickens. Poultry Science, 83, 1358.
van der Klis, J.D., Versteegh, H.A.J., Simons, P.C.M. & Kies, A.K. (1997) The efficacy of phytase in corn-soybean meal-based diets for laying hens. Poultry Science, 76, 1535- 1542.
Weurding R.E., Enting, H. and Verstegen, M.W.A. (2002) The relation between starch digestion rate and amino acid level in broiler chicken nutrition. PhD Thesis Wageningen Institute of Animal Sciences.
Wu, Y.B., Ravindran, V. & Hendriks, W.H. (2003) Effects of microbial phytase, produced by solid-state fermentation, on the performance and nutrient utilisation of broilers fed maize- and wheat-based diets. British Poultry Science, 44, 710-718.
Wu, Y.B., Ravindran, V., Thomas, D.G., Birtles, M.J. & Hendriks, W.H. (2004) Influence of phytase and xylanase, individually or in combination, on performance, apparent metabolisable energy, digestive tract measurements and gut morphology in broilers fed wheat-based diets containing adequate level of phosphorus. British Poultry Science, 45, 76-84.
Zanella I., Sakomura N.K., Rosa A.P., Figueiredo A.N. & Magon L. (2004) Enzyme supplementation on digestibility of corn-soybean diets for broiler chicks. Ars Veterinaria 20, 144-150.
Further Reading
| - | You can view other papers presented at the Carolina Poultry Nutrition Conference 2008 by clicking here. |
May 2009








