



Enzyme Combinations to Optimise By-Products Use in Corn-Based Poultry Feed
As more by-products are included in diets for poultry, there are greater opportunities to maximise nutrient availability by the use of feed enzymes. Dr Janet C. Remus from Danisco Animal Nutrition reviewed this topic, focussing on bakery by-products, wheat by-products and distillers dried grains, for the 35th Carolina Poultry Nutrition Conference last year.Introduction
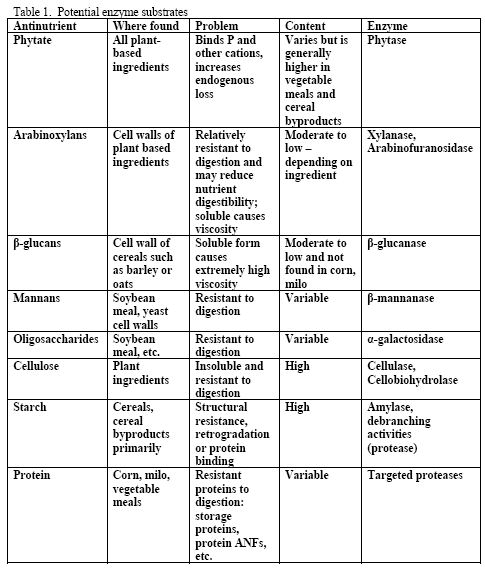
Although there is a variety of potential substrates for enzymes in poultry diets (see Table 1), the primary ones generally targeted in practical corn-based diets are phytate, arabinoxylans and starch. Mannans and oligosaccharides may also be potential substrates but dietary content of these may be more variable across ingredients than the aforementioned three. Beta-glucans are normally considered when feeding viscous cereals such as barley or oats as the primary cereal or at levels at or above 10 per cent of the diet for either cereal. Due to the level of beta-glucans and the insolubility of these non-starch polysaccharides (NSPs) in typical US ingredients used in corn-based diets, beta-glucans are not normally a NSP of concern for most diets. Cellulose is not a practical target in poultry as no enzyme system currently exists that would efficiently and cost-effectively fully release glucose from this NSP within the constraints of the bird’s gastrointestinal tract.
Enzyme use is well documented across different types of poultry diets. Example papers on amylase (Ritz et al., 1995; Jiang et al., 2008), protease (Ghazi et al., 2002; Ghazi et al., 2003; Wang et al., 2008), xylanase (Mathlouthi et al., 2002; Cowieson et al., 2005), beta-glucanase (Mathlouthi et al., 2002), mixes of two or more of the aforementioned activities (Pettersson and Åman, 1992; Vranješ et al., 1994; Zanella et al., 1999; Hong et al., 2002; Mathlouthi et al., 2002; Meng et al., 2005; Cowieson and Ravindran, 2008a, b) as well as phytase (Onyango et al., 2005; Liu et al., 2008a, b) are among the many that can be found in the scientific literature.
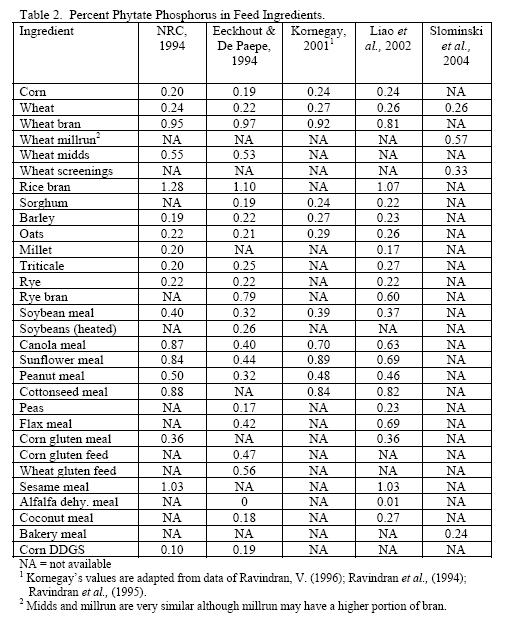
However, trials often examine one type of enzyme in isolation. For example, many of the published phytase papers do not examine relationships between phytase and various carbohydrases or proteases in practical diets. Given that many cereals, cereal by-products or vegetable protein by-products can vary in phytate (Table 2) as well as NSPs (Tables 3, 4, 5), it would seem logical that combinations of phytase, non-starch polysaccharidases (NSPases) or other activities may provide a benefit in practical diets.
Some recent papers published in the poultry research press have examined phytase with and without carbohydrase inclusion in corn-based diets (Cowieson and Adeola, 2005; Olukosi et al., 2007) or wheat-based diets (Ravindran, 2001; Wu et al., 2004). From a practical standpoint, many commercial companies in the poultry business are using phytase currently so demonstration of carbohydrase or protease efficacy in the presence of a phytase has become more important. Although many enzyme trials have focused on simple cereal/soy diets (for example: Wu et al., 2004; Cowieson et al., 2005; Olukosi et al., 2007; Cowieson et al., 2008a; Jiang et al., 2008), the responses noted with enzyme addition have ramifications for diets containing by-products as these diets may have higher levels problem substrate(s) present. There are published papers on corn-based diets containing several different by-products or vegetable meals, these provide evidence of enzyme efficacy (Dipeolu et al., 2005; Yuan et al., 2008). Unfortunately, there are a limited number of trials that have looked at inclusion of only one byproduct in the presence of feed enzymes.
Vegetable By-Products and Anti-Nutritional Issues
Typical by-products used in feed formulation can vary widely by region. But in general, typical problems involve NSPs, phytate, starch and amino acids where plant-based by-products are concerned. In these ingredients, digestibility and anti-nutritional factors are almost always involved in the decision process of when and how much to use to help reduce cost while not jeopardising performance. Toxic anti-nutritional factors such as gossypol, mycotoxins, etc. are obvious limiting factors. But issues on NSP-related effects on nutrient uptake, gastrointestinal passage rate and subsequent bird performance to problems in starch, amino acid or phosphorus (mineral) availability are also key in the decision process. It should be noted in any discussion on usage of by-product, particularly at higher levels, that good ingredient quality monitoring programmes should be in place.
Non-starch polysaccharides
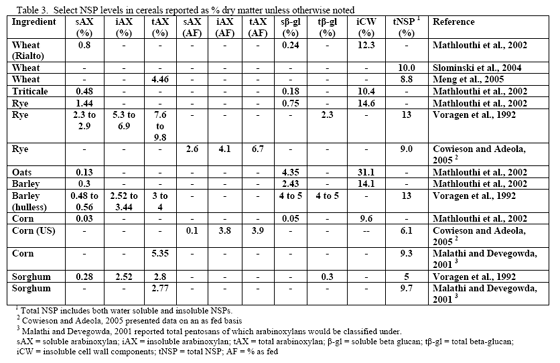
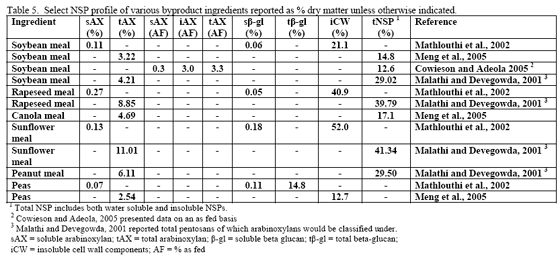
Non-starch polysaccharide issues are best addressed by the appropriate NSPase (table 1). However it is fair to note that the degree of and type of benefit of NSP enzymes may depend on the nature of the NSP present, i.e. soluble (Mathlouthi et al., 2002) or insoluble (Jaroni et al., 1999), or even a factors like particle size (Mavromichalis et al., 2000; Aulrich and Flachowsky, 2001). In addition, heat processing can affect physical characteristics of NSPs (Cowieson et al., 2005; González-Alvarado et al., 2008) Viscous fibre prolongs gastric emptying and slows transit time (Malkki, 2001), which in growing animals alters nutrient digestibility and generates performance losses. For corn-based diets, insoluble NSPs predominate in many of the ingredients typically used (see tables 3, 4 and 5).
Insoluble fibre/NSPs can affect gut transit time, gut motility and may also hinder the ability of endogenous enzymes to gain access to their respective substrates (Choct, 2001). Insoluble NSPs do not cause viscosity but these cell-wall components can encapsulate nutrients inside intact cell walls. Correspondingly, the finer the grind or particle size, the more of these encapsulated nutrients may already be released. Another way of addressing intact cell walls is to add an NSPase such as xylanase to the feed, with the intention of this enzyme acting to open intact cell walls via its action on the NSP arabinoxylan, thus allowing endogenous enzymes access to previously ‘hidden’ nutrients.
The mechanism of action may be related to the ability of insoluble fibre to ‘bind’ or ‘hold’ water, thus influencing gut bulk (physical fill) and potentially motility. Some consider wheat bran or rice bran as possibly beneficial in laxation for humans (Dikeman et al., 2006), although this is typically not the reason for use in poultry diets. In vitro tests on water-binding and water-holding capacity of various feed ingredients have noted differences between major feedstuffs. In general, corn was found to have lower water holding capacity than does soybean meal or other protein meals whereas potato flakes were found to have the highest water holding capacity (Partridge, 2001). All ingredients, but grass meal and sunflower meal, responded with reductions in water holding capacity greater than 10 per cent when a carbohydrase/protease product was added to the in vitro test system (Partridge, 2001). Aulrich and Flachowsky (2001) examined the relationship between particle size and water- binding as well as water-holding capacity of wheat bran. These authors found that as particle size went from 1 mm to 0.25 mm, there was a reduction in both water-binding and water-holding capacity of wheat bran. In addition, if an NSPase mix of xylanase and β-glucanase were applied to the wheat bran, a linear reduction in water-binding and water-holding capacity was noted with increasing enzyme inclusion (Aulrich and Flachowsky, 2001). Interestingly enough, these authors also noted in the porcine in vitro stomach and small intestine simulations that NSPase supplementation improved release of ‘bound’ protein in wheat bran.
Since nutrient flow to the villi on the gut wall depends on gut motility and corresponding convective movement of water and nutrients, insoluble fibre may affect feed intake, nutrient absorption and ultimately bird performance. So in this scenario, NSP enzymes act to alleviate the negative water-binding and water-holding effects of insoluble fibre/NSPs in the gastrointestinal tract, which in turn helps to reduce more normal transit time and nutrient flow. A fairly extreme example of how insoluble NSPs can affect growth of the bird in the absence of NSPases can be seen in the work of Pettersson and Åman, 1992. These authors used a diet containing 68 per cent oat bran or extracted oat bran with or without NSPase addition and noted dramatic improvement in chick performance with enzyme addition even considering that they pair-fed one of the treatment pairs (see table 6).
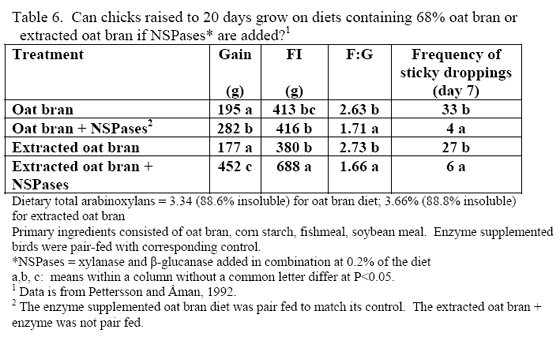
Phytate
Phytic acid has long been known as an antinutrient due to the presence of bound phosphorus on its structure but also its ability to bind positively charged substances (e.g. Kornegay, 2001 or similar articles for review) and alter secretion of endogenous enzymes (Dilworth et al., 2004; Liu et al., 2008 b). High phytate levels depressed chicks weights and G:F (Liu et al., 2008 b) as well as nutrient digestibility (Ravindran et al., 2006). Plus high phytate reduced activity of disaccharidases and Na+K+ATPase in the duodenum (Liu et al., 2008 b).
The mode of action of phytate in increasing endogenous nutrient loss has become clearer over the last five years, which helps explain why phytase may affect nutrients other than those that could be directly bound to phytate. In addition, new research suggests that phytase addition to nutritional marginal diets may help improve lymphocyte numbers as well as antibodies in the sera and mucosa of broilers (Liu et al., 2008a).
In all, it should be realised that the higher the dietary phytate level, the greater the potential for phytate to generate digestive issues and potentially increase maintenance cost of digestion. Use of phytase helps alleviate issues with this substrate and is known to generate feed cost savings via lower inclusion of inorganic phosphorus. However, it should be considered that higher phytate diets have greater potential for negative effects of this substrate, so it may be beneficial to consider higher phytase inclusion per ton feed to help alleviate these effects.
Starch
Starch is the easiest of the carbohydrates to digest but is not fully utilised by poultry at the terminal ileum. Noy and Sklan (1994) estimated that 11 to 18 per cent of the starch may be undigested at the terminal ileum in chicks of 4 to 21 days of age. However, crystalline structure, component ratio of amylase to amylopectin, protein binding, cell wall encapsulation or even gelatinisation can all affect how efficiently starch is digested by the bird. Cowieson (2005) reviewed these factors for corn and Weurding and coworkers (2001a, b) noted clear differences in the rate and extent of starch digestion for various feed ingredients.
In cereal by-products, starch content is often highly variable and may vary widely in quality. In bakery by-products, care must be taken by the manufacturer to ensure that already cooked items containing starch are not retrograded during the second cooking process. In addition, there is potential for the formation of Maillard reaction products between glucose and amino acids such as lysine, although this depends on the type of bakery goods included in the mix and the content of reactive materials. Maillard reaction products are also a great concern in distillers dried grains with solubles (DDGS). Partially reacted products will assay as lysine but are not wholly bioavailable as lysine. The amino acid lysine is a concern here due to it having two amino groups that can be reactive whereas other amino acids, except proline, have one amino group. Regrettably, there is no enzyme that will counteract the effect of the Maillard reaction on lysine after the Schiff base step.
Protein anti-nutritional factors
For poultry feed, proteases may target issues in vegetable protein meals, protein anti-nutritional factors (ANFs) or storage proteins found in plant-origin materials. It is known that a targeted protease(s) can be used to help degrade lectins, trypsin inhibitors (Hessing et al., 1995) and other protein ANFs (Ghazi et al., 2002). Proteases also may be used to target storage proteins. These proteins ‘store’ or bind starch and are one possible cause of starch that is resistant to digestion (Brown, 1996).
Vegetable By-Products: Where Are We Now?
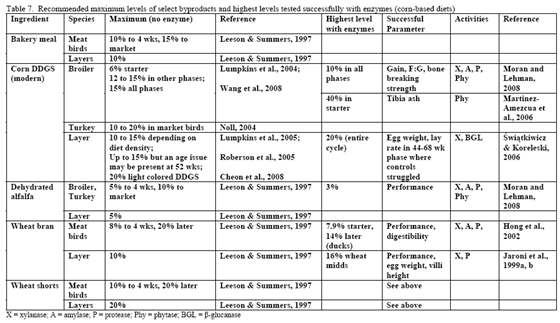
Table 7 shows general recommendations for select by-products that may be encountered in the field. Some of these are discussed further in this paper but the reader is strongly encouraged, when using higher levels of by-products, to have a strong quality control programme in place to help ensure successful usage. In addition, it may be helpful to evaluate what anti-nutritional factors that by-product(s) may be bringing into the diet particularly as level increases. This can help make the decision of the type of enzyme(s) that may be needed to remedy digestive and performance issues clearer. Relatively few trials have examined enzyme use in high by-product type diets. Logically, this may be due historic need to ‘prove’ response in what was the status quo type diet – simple corn/soy. Some by-products merit a few extra words as these typically exhibit high variance.
Bakery by-products
As a whole, bakery byproducts can vary considerably in composition depending on the relative sources of raw material around the production plant. Typical bakery by-product raw materials may consist any of the following: raw doughs, partially cooked doughs, doughnuts, breads, muffins, cookies, crackers, candy, snack foods, chips, cakes, inedible flour, unsalable nuts, etc.
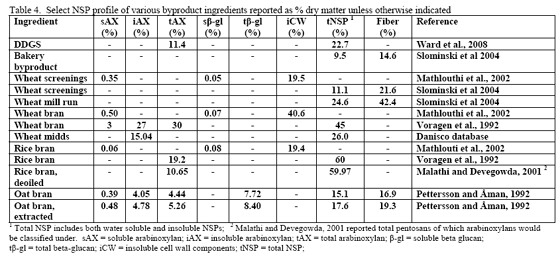
Since the component ingredients vary in composition, the final bakery by-product will vary from plant to plant. In their examination of bakery product originating from different feed manufacturers, Slominski and co-workers (2004) noted that high variability existed in starch fat, NSP and phytate phosphorus. These authors noted a difference in NSP content ranging from 3.3 to 17.0 per cent in the 12 samples analysed (mean = 8.7 per cent with a standard deviation of 5.53 and coefficient of variation of 63.6 per cent). Total fibre showed the highest variance, although the authors only analysed eight of the 12 samples. The range in total fibre was 6.9 to 32.7 per cent with a SD of 9.57 and CV of 71.4 per cent. Phytate phosphorus content ranged from 0.03 to 0.47 per cent (mean = 0.22 per cent, SD = 0.15 and CV of 69.5 per cent). For starch, the range ran from 24.7 to 49.3 per cent with a mean of 37.8 (SD of 8.29 and CV of 21.9 per cent). Fat content ranged from 4.2 to 10.2 per cent with a mean of 8.0 per cent (SD of 18.2 and CV of 22.7 per cent). Protein was less variable with a mean of 11.9 per cent (SD of 1.35, CV of 11.3 per cent). Dry matter was very consistent at 91.6 (SD of 9.8, CV of 1.06 per cent). There was an 85 per cent correlation between NSP and starch wherein increases in NSP meant reduced starch levels in bakery meal (Slominski et al., 2004). No relevant correlation was present for starch versus fat in this by-product.
Since it is typical that xylanases and/or amylases are added in the mixing of doughs to enhance the production process, it is an open question as to how little or much these processing enzymes have improved nutrient value of components in bakery meal. Much depends on type of bakery product as raw or partially cooked doughs from bread or pizza crust would logically have different potential for NSPs and starch availability from cakes, cookies and candies.
The potential for bakery as an energy source in poultry diets is clear. However, users should monitor proximate composition, salt and similar nutrients. But also it may be helpful to periodically measure NSP or at least NDF and ADF content of incoming bakery by-product from each of their suppliers’ plants.
Wheat by-products
Traditionally, wheat by-products are been restricted to diets for layers, breeders, ducks or older turkeys due to concern about fibre and starch contents although Leeson and Summers (1997) suggest that wheat by-products may be used at up to 10 per cent of diet in young birds. Wheat mill run (similar to midds but with more bran), for example, contains about 22.4 per cent total NSPs (Slominski et al., 2004; CV of 13.2 per cent on six samples). The bulk of which are insoluble NSP rather than the viscosity-causing soluble NSP. Phytate P levels also run high in wheat mill run at 0.52 per cent (Slominski et al., 2004). Like wheat midds, starch and fat contents are the most variable components. Starch averaged 26.4 per cent (SD of 5.24, CV of 19.8 per cent) whereas fat had a mean of 4.3 per cent (SD of 1.02, CV of 23.7 per cent; Slominski et al., 2004).
The challenge with any of the wheat by-products is that the starch, NSP level and perhaps even phytate can vary considerably from flour mill to flour mill. So again, monitoring programmes are essential with this byproduct. In table 7, corn-based diets containing midds and feed enzymes have been successfully used in duck and layer to aid bird performance.
DDGS
For the US, DDGS is mainly made from corn. In Canada, the cereal used in fuel ethanol production can be wheat, corn or blends of the two depending on location. In their NSP screen of US-origin DDGS from modern ethanol plants, Ward and co-workers (2008) noted that arabinoxylans and cellulose were the predominant NSPs. The reported value of 11.4 per cent arabinoxylans (dry matter basis) from Ward and co-workers (2008) agrees with that of the Danisco database which averages 11.7 per cent total arabinoxylans. Insoluble NSPs predominated in US DDGS for all NSPs measured but fucose and ribose (Ward et al., 2008).
Lysine is of special concern in this ingredient as it can irreversibly react with starch via the Maillard reaction, which renders the reacted product unavailable to the bird. A screen of 20 Minnesota-origin DDGS samples revealed that while mean lysine digestibility averaged 72 per cent, the range was between 59 and 84 per cent (Parsons, 2006). In a related project examining if phosphorus availability could be increased in DDGS, amino acid availability was also examined. Autoclaving or oven drying DDGS reduced lysine availability although it did improve phosphorus availability (Parsons, 2006). Thus lysine deserves special consideration when formulating diets with DDGS as enzyme supplementation will not restore availability of Maillard-reacted lysine. As with other by-products, use of digestible amino acids in formulation is strongly recommended.
Use of DDGS in poultry feeds has varied by age and species of the bird in question (Table 7 or see the University of Minnesota's DDGS web site). In pelleted feeds for broilers, DDGS has been associated with reduced pellet quality at levels greater than 15 per cent (Wang et al., 2008). Particle size of the DDGS, starch content as well as its oil content affect pellet quality, so all should be considered when using higher levels of DDGS as poorer pellet quality can impact performance.
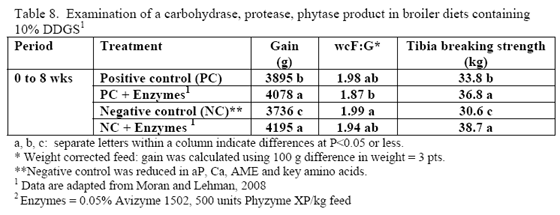
Research is relatively limited on how NSPases and/or phytase may impact the nutritional value of diets containing this by-product. Martinez-Amezcua and coworkers (2006) noted that 1,000 or 10,000 units phytase/kg feed improved tibia ash and threonine digestibility of broiler chicks fed a corn-based diet with 40 per cent DDGS but not apparent metabolisable energy (AME). Work by Moran and Lehman (2008) using xylanase, amylase, protease and phytase supplementation to a corn/soy/10 per cent DDGS diet for broilers raised to 56 days of age noted improvements in weight gain, weight-corrected feed conversion (F:G) as well as bone-breaking strength in these birds (Table 8). For laying hens, some trials have reported reduced egg production with feeding 15 to 20 per cent DDGS in older layers (Roberson et al., 2006, Swiatkiwicz & Koreleski, 2006). Addition of NSPases in the 44- to 68-week feed phase helped offset the drop in lay rate and daily egg mass noted by Swiatkiwicz and Koreleski (2006) in diets with 20 per cent DDGS versus the non-supplemented diet with 20 per cent DDGS.
The type of fermentation process and possible use of fractionation present in the fuel ethanol industry means the user needs to be certain of the type of corn DDGS they are receiving as the new fractionation products differ in nutrient profile (Kim et al., 2008). Monitoring other key factors such as protein, fat, starch, fibre, ash, dry matter, total and bioavailable amino acids (particularly lysine), sodium, phosphorus, phytate and sulphur is strongly recommended. Mycotoxin monitoring is also recommended as those from the corn itself are not destroyed in the fermentation process. Plus, mould growth due to improper storage or drying can occur. Periodic analysis of NSP profile may also be helpful particularly as the productions methods (and fractionation) evolve over the next few years.
Closing Thoughts
As higher levels of by-products are used in diets, problem substrates correspondingly will increase. As enzymes typically have demonstrated efficacy in simple cereal diets, these benefits should continue as target substrates increase for key enzyme activities. However, the user needs to be aware of just what those substrates are and how much is coming into their diets to determine the appropriate enzyme or enzymes use. Regrettably, several key common by-products are highly variable in composition, which means good ingredient monitoring systems need to be in place and that targeted enzymes may have a role to help maintain nutritional uniformity.
References
Aulrich, K. and Flachowsky, G. 2001. Studies on the mode of action of non-starch polysaccharides (NSP)-degrading enzymes in vitro 2. Communication: Effects on nutrient release and hydration properties. Archives of Animal Nutrition 54: 19-32.
Brown, I. 1996. Complex carbohydrates and resistant starch. Nutrition Reviews 115-119.
Cheon, Y.J., Lee, H.L., Shin, M.H., Jang, A., Lee, S.K., Lee, J.H., Lee, B.D. and Son, C.K. 2008. Effects of corn distiller’s dried grains with solubles on production and egg quality in laying hens. Asian-Australia Journal of Animal Science 21: 1318-1323.
Choct, M. 2001. Enzyme supplementation of poultry diets based on viscous cereals. In: Enzymes in Farm Animal Nutrition (Eds: M.R. Bedford and G.G. Partridge) p145-160. CAB International.
Cowieson, A.J. 2005. Factors that affect the nutritional value of maize for broilers. Animal Feed Science and Technology 119: 293-305.
Cowieson, A.J. and Adeola, O. 2005. Carbohydrases, protease, and phytase have an additive beneficial effect in nutritional marginal diets for broiler chicks. Poultry Science 84: 1860- 1867.
Cowieson, A.J., Hruby, M. and Faurschou Isaksen, M. 2005. The effect of conditioning temperature and exogenous xylanase addition on the viscosity of wheat-based diets and the performance of broiler chickens. British Poultry Science 46: 717-724.
Cowieson, A.J. and Ravindran, V. 2008a. Effect of exogenous enzymes in maize-based diets varying in nutrient density for young broilers: growth performance and digestibility of energy, minerals and amino acids. British Poultry Science 49: 37-44.
Cowieson, A.J. and Ravindran, V. 2008b. Sensitivity of broiler starters to three doses of an enzyme cocktail in maize-based diets. British Poultry Science 49: 340-346.
Dikeman, C.L., Murphy, M.R. and Fahey, G.C. Jr. 2006. Dietary fibers affect viscosity of solutions and simulated human gastric and small intestinal digesta. Journal of Nutrition 136: 913-919.
Dilworth, L.L., Omoruyi, F.O., Simon, O., Morrison, E.Y. and Asemota, H.N. 2004. Hypoglycemia and faecal minerals in rats fed phytate. Nutr. Food Sci. 34: 60-64.
Dipeolu, M.A., Eruvbetine, D., Oguntona, E.B., Bankole, O.O. and Sowunmi, K.S. 2005. Comparison of effects of antibiotics and enzyme inclusion in diets of laying birds. Archivos de Zootecnia 54: 3-11.
Eeckhout, W. and M. De Paepe. 1994. Total phosphorus, phytate-phosphorus and phytase activity in plant feedstuffs. Anim. Feed Sci. Tech. 47:19-29
Ghazi, S., Rooke, J.A., Galbraith, H. and Bedford, M.R. 2002. The potential for the improvement of the nutritive value of soya-bean meal by different proteases in broiler chicks and broiler cockerels. British Poultry Science 43: 70-77.
Ghazi, S., Rooke, J.A., Galbraith, H. 2003. Improvement of the nutritive value of soybean meal by protease and α-galactosidase treatment in broiler cockerels and broiler chicks. British Poultry Science 44: 410-418.
González-Alvardo, J.M., Jiménez-Moreno, E., Valencia, D.G., Lázaro, R. and Mateos, G.G. 2008. Effects of fiber source and heat processing of the cereal on development and pH of the gastrointestinal tract of broilers fed diets based on corn or rice. Poultry Science 87: 1779- 1795.
Hessing, M., Mocking-Bode, H., Bleeker-Marcelis, H., Van Laarhoven, H., Rooke, J.A. and Morgan, A.J. 1995. Quality of soybean meals and effect of microbial enzymes in degrading soya antinutitional compounds (ANCs) using immunochemical, microscopic techniques and in vivo studies. In: 2nd European Symposium on Feed Enzymes (eds: W. van Hartingsveldt, M. Hessing, J.P. van der Lugt and W.A.C. Somers) pgs 176-177. TNO Nutrition and Food Research Institute, Zeist.
Hong, D., Burrows, H. and Adeola, O. 2002. Addition of enzymes to starter and grower diets for ducks. Poultry Science 81: 1842-1849.
Jaroni, D., Scheideler, S.E., Beck, M. and Wyatt, C. 1999a. The effect of dietary wheat middlings and enzyme supplementation. 1. Late egg production efficiency, egg yields, egg composition in two strains of Leghorn hens. Poultry Science 78: 841-847.
Jaroni, D., Scheideler, S.E., Beck, M. and Wyatt, C. 1999b. The effect of dietary wheat middlings and enzyme supplementation. II. Apparent nutrient digestibility, digestive tract size, gut viscosity, and gut morphology in two strains of Leghorn layers. Poultry Science 78: 1664-1674.
Jiang, Z., Zhou, Y., Lu, F., Han, Z. and Wang, T. 2008. Effects of different levels of supplementary alpha-amylase on digestive enzyme activities and pancreatic amylase mRNA expression of young broilers. Asian-Austrialian Journal of Animal Science 21: 97-102.
Kim, E.J., Martinez Amezcua, C., Utterback, P.L. and Parsons, C.M. 2008. Phosphorus bioavailability, true metabolizable energy, and amino acid digestibilities of high protein corn distillers dried grains and dehydrated corn germ. Poultry Science 87: 700-705.
Kornegay, E.T. 2001. Digestion of phosphorus and other nutrients: the role of phytases and factors influencing their activity. In: Enzymes in Farm Animal Nutrition (Eds Bedford, M.R. and G.G. Partridge). Pgs: 237-271. CAB International.
Liao, S.F., W.C. Sauer, A.K. Kies. 2002. Supplementation of microbial phytase to swine diets: Effect on utilization of nutrients. Pages 199-227. In: Food Science and Product Technology. Nakano, T. and L. Ozimek (Eds.) Research Signpost, Trivandrum, India.
Leeson, S. and Summers, J.D. 1997. In: Commercial Poultry Nutrition, 2nd edition. University Books, Guelph, Ontario.
Liu, N., Ru, Y.J., Cowieson, A.J., Li, F.D. and Cheng, X.CH. 2008a. Effects of phytate and phytase on the performance and immune function of broilers fed nutritionally marginal diets. Poultry Science 87: 1105-1111.
Liu, N., Ru, Y.J., Li, F.D. and Cowieson, A.J. 2008b. Effect of diet containing phytate and phytase on the activity and mRNA expression of carbohydrase and transporter in chickens. Journal of Animal Science published online on August 15, 2008 as doi: 10.2527/jas.2008-1234
Lumpkins, B.S. Batal, A.B. and Dale, N.M. 2004. Evaluation of distillers dried grains with solubles as a feed ingredient for broilers. Poultry Science 83: 1891-1896.
Lumpkins, B., Batal, A. and Dale, N. 2005. Use of distillers dried grains plus solubles in laying hen diets. Journal of Applied Poultry Research 14: 25-31.
Maenz, D.D., Engele-Schaan, C.M. and Classen, H.L. 1998. Phytase activity in the small intestinal brush border membrane of the chicken. Poultry Science 77: 557-563.
Malathi, V. and Devegowda, G. 2001. In vitro evaluation of nonstarch polysaccharide digestibility of feed ingredients by enzymes. Poultry Science 80: 302-305.
Malkki, A. 2001. Physical properties of dietary fiber as keys to physiological functions. Cereal Foods World 46: 196-199.
Martinez-Amezcua, C., Parsons, C.M. and Baker, D.H. 2006. Effect of microbial phytase and citric acid on phosphorus bioavailability, apparent metabolizable energy, and amino acid digestibility in distillers dried grains with solubles in chicks. Poultry Science 85: 470-475.
Mathlouthi, N., Saulnier, L., Quemener, B. and Larbier, M. 2002. Xylanase, β-glucanase, and other side enzymatic activities have greater effects on viscosity of several feedstuffs than xylanase or β-glucanase used alone or in combination. Journal of Agricultural and Food Chemistry 50: 5121-5127.
Mavromichalis, I., Hancock, J.D., Senne, B.W., Gugle, T.L., Kennedy, G.A., Hines, R.H. and Wyatt, C.L. 2000. Enzyme supplementation and particle size of wheat in diets for nursery and finishing pigs. Journal of Animal Science 78: 3086-3095.
Meng, X., Slominski, B.A., Nyachoti, C.M., Campbell, C.M. and Guenter, W. 2005. Degradation of cell wall polysaccharides by combinations of carbohydrase enzymes and their effect on nutrient utilization and broiler chicken performance. Poultry Science 84: 37-47.
Moran, E.T. and Lehman, R. 2008. Response to combined amylase-phytase-protease-xylanase supplementation when 8-week broiler males had received corn-soybean meal feeds devoid of antimicrobials with/without alfalfa and/or DDGS. International Poultry Science Form Proceedings 2008 p 26.
National Research Council. 1994. Composition of feedstuffs used in poultry diets. Pages 62- 65. In: Nutrient Requirements of Poultry, 9th edition, National Academy Press, Washington D.C.
Noll, S.L., Stangeland, V., Speers, G., Parsons, C.M. and Brannon, J. 2004. Feeding DDGS to market turkeys. Multi-State Poultry Nutrition and Feeding Conference, May 25-27, 2004.
Noy, Y. and Sklan, D. 1994. Digestion and absorption in the young chick. Poultry Science 73: 366-373.
Oluskosi, O.A., Cowieson, A.J. and Adeola, O. 2007. Age-related influence of a cocktail of xylanase, amylase, protease or phytase individually or in combination in broilers. Poultry Science 86: 77-86.
Onyango, E.M., Bedford, M.R. and Adeola, O. 2005. Efficacy of an evolved Escherichia coli phytase in diets of broiler chicks. Poultry Science 84: 248-255.
Parsons, C.M., Martinez, C., Singh, V., Radhakrishman, S. and Noll, S. 2006. Nutritional value of conventional and modified DDGS for poultry. Multi-State Poultry Nutrition and Feeding Conference.
Pettersson, D. and Åman, P. 1992. Production responses and serum lipid concentration of broiler chickens fed diets based on oat bran and extracted oat bran with and without enzyme supplementation. J Sci Food Agric 58: 569-576.
Partridge, G.G. 2001. The role and efficacy of carbohydrase enzymes in pig nutrition. In: Enzymes in Farm Animal Nutrition (Eds: M.R. Bedford and G.G. Partridge) pgs 161-198. CAB International.
Ravindran, V. 1996. Occurrence of phytic acid in plant feed ingredients. Pages 85-92. In: Phytase in Animal Nutrition and Waste Management. (Eds: Coelho, M.B. and E.T. Kornegay) BASF Corporation, Mount Olive, NJ.
Ravindran, V., Morel, P.C.H., Partridge, G.G., Hruby, M. and Sands, J.S. 2006. Influence of an Escherichia coli-derived phytase on nutrient utilization in broiler starters fed diets containing varying concentrations of phytic acid. Poultry Science 85: 82-89.
Ravindran, V., G. Ravindran and S. Sivalogan. 1994. Total and phytate phosphorus contents of various foods and feedstuffs of plant origin. Food Chem. 50: 133-136.
Ravindran, V., W.L.O. Bryden and E.T. Kornegay. 1995. Phytates: occurrence, bioavailability and implications in poultry nutrition. Poult. Avian Biol. Rev. 6:125-143.
Ravindran, V. 2001. Enzyme combinations to improve the nutritive value of feed ingredients. Zootecnica International 2: 32-35.
Ritz, C.W., Hulet, R.M., Self, B.B. and Denbow, D.M. 1995. Growth and intestinal morphology of male turkeys as influenced by dietary supplementation of amylase and xylanase. Poul. Sci. 74: 1329-1334.
Roberson, K.D., Kalbfleisch, J.L., Pan, W. and Charbenau, R.A. 2005. Effect of corn distiller’s dried grains with solubles at various levels on performance of laying hens and egg yolk color. International Journal of Poultry Science 4: 44-51.
Slominski, B.A., Boros, D., Campbell, L.D., Guenter, W. and Jones, O. 2004. Wheat byproducts in poultry nutrition. Part 1. Chemical and nutritive composition of wheat screenings, bakery byproducts and wheat mill run. Can.J.Anim.Sci. 84: 421-428
Swiatkiwicz, S. and Koreleski, J. 2006. Effect of maize distillers dried grains with solubles and dietary enzyme supplementation on the performance of laying hens. Journal of Animal and Feed Sciences 15: 253-260.
Vranješ, M.V., Pfirter, H.P., Wenk, C. 1994. Influence of processing treatment and type of cereal on the effect of dietary enzymes in broiler diets. Animal Feed Science and Technology 46: 261-270.
Voragen, A.G.J., Gruppen, H., Verbruggen, M.A. and Viëtor, R.J. 1992. Characterization of cereal arabinoxylans. In: Xylans and Xylanases (Eds J. Visser et al), pgs 51-67. Elsevier Science
Wang, H., Guo, Y. and Shih, J.C.H. 2008. Effects of dietary supplementation of keratinase on growth performance, nitrogen retention and intestinal morphology of broiler chickens fed diets with soybean meal and cottonseed meal. Animal Feed Science and Technology 140: 376-384.
Wang, Z., Cerrate, S., Coto, C., Yan, F., Costa, F.P., Abdel-Maksoud, A. and Waldroup, P.W. 2008. Evaluation of corn distillers dried grains with solubles in broiler diets formulated to be isocaloric at industry energy levels or formulated to optimum density with constant 1 per cent fat. International Journal of Poultry Science 7: 630-637.
Ward, N.T., Zijlstra, R.T., Parsons, C. and Starkey, C. 2008. Non-starch polysaccharide (NSP) content of US commercial corn distiller’s dried grains with solubles (DDGS). Poultry Science 87(Suppl 1): 39.
Weurding, R.E., Veldman, A., Veen, W.A.G., van der Aar, P.J. and Verstegen, M.W.A. 2001a. Journal of Nutrition 131: 2329-2335.
Weurding, R.E., Veldman, A., Veen, W.A.G., van der Aar, P.J. and Verstegen, M.W.A. 2001b. Journal of Nutrition 131: 2336-2342.
Wu, Y.B., Ravindran, V., Thomas, D.G., Birtles, M.J. and Hendriks, W.H. 2004. Influence of phytase and xylanase, individually or in combination, on performance, apparent metabolisable energy, digestive tract measurements and gut morphology in broilers fed wheat-based diets containing adequate level of phosphorus. British Poultry Science 45: 76- 84.
Yuan, J., Yao, J., Yang, F., Yang, X., Wan, X., Han, J., Wang, Y., Chen, X., Liu, Y., Zhou, Z., Zhou, N. and Feng, X. 2008. Effects of supplementing different levels of a commercial enzyme complex on performance, nutrient availability, enzyme activity and gut morphology of broilers. Asian-Australian Journal of Animal Science 21: 692-700.
Zanella, I., Sakomura, N.K., Silverside, F.G., Fiqueirdo, A. and Pack, M. 1999. Effect of enzyme supplementation of broiler diets based on corn and soybeans. Poultry Science 78: 561-568.
Further Reading
| - | You can view other papers presented at the Carolina Poultry Nutrition Conference 2008 by clicking here. |
May 2009








