



Antibiotic-free and reduced antibiotic use in broiler production
History, developments and challenges. An Aviagen Focus SeriesBackground
Antibiotics (AB) are a type of antimicrobial medication used to treat infections caused by bacteria. In the 1930s, a sulfonamide was the first commercially available product to treat infections caused by Gram- positive bacteria. In the mid-1940s, penicillin was introduced on a large scale marking the beginning of the AB era. Following their discovery and development into products for human and animal health, multiple AB have been used to treat bacterial infections by either preventing the formation of the cell wall, which effectively kills the bacteria (bactericidal effect), or stopping their replication (bacteriostatic effect).
Antibiotic Resistance
Antibiotic resistance (ABR) results when a bacterial organism develops the ability to resist the effects of one or multiple AB to which they were once sensitive. As a result, diseases caused by ABR bacteria are difficult to treat, leading to persistent infection, severe clinical signs, higher treatment costs and increased mortality. ABR is an increasingly serious global threat to both public and animal health, and therefore, it is widely recognized that steps must be taken to reduce the use of AB while allowing their use in a judicious manner and preserving their continuous efficacy.
ABR is an ancient, naturally occurring phenomenon that evolved long before AB were available to treat human or animal diseases. In 2011, a study published in Nature (477:457-461), reported the identification of various genes in bacteria recovered from 30,000-year-old samples of permafrost (collected in the Yukon Territory, Canada), encoding resistance to ꞵ-lactam, tetracycline, and glycopeptide AB, including a vancomycin-resistance gene similar to modern variants. ABR can be described as a type of microbial warfare, by which some microorganisms gain a selective advantage by producing their own AB that either inhibit or eliminate other competing bacteria. Although ABR occurs naturally, numerous studies have highlighted that lack of research, difficulties developing new AB, inadequate disease prevention and misuse in humans and animals are accelerating the process.
ABR can be spread through bacterial reproduction or through the transfer of genes between different bacteria in the same location. Mutations in some bacteria have resulted in the production of enzymes that can inactivate some AB or the development of alternate cellular functions that circumvent them. In other cases, genes for resistance can change the bacterial cell wall structure, making it impenetrable by AB. Resistance has been found in pathogenic as well as in commensal (normal intestinal) and environmental bacteria. Many studies have demonstrated the emergence and spread of multi-antibiotic or multi-drug resistant (MDR) bacteria “superbugs” in both human and animal populations, which are difficult and, in some cases, impossible to treat.
Antibiotic Resistance and Foodborne Illness
The development and increasing frequency of ABR has led to concerns about foodborne diseases caused by enteric pathogens. Enteric bacteria such as Salmonella and Campylobacter, commonly found in poultry, can spread to people through contaminated food products. Campylobacter is a leading foodborne pathogen carried in the intestinal tract of a wide range of domestic and farm animals, including poultry, wild animals and birds. A growing number of Campylobacter resistant strains (due to mutations in the bacteria chromosome), especially against fluoroquinolones (ciprofloxacin) and macrolides (erythromycin, azithromycin, clarithromycin), have been reported in the USA, Canada and Europe. Other enteric bacteria contain enzymes in a group called extended-spectrum ꞵ-lactamase (ESBL) that allows them to become resistant to various penicillins and cephalosporins, generally mediated by transmissible extrachromosomal circular DNA elements known as plasmids. Non-typhoidal Salmonella species can be MDR against ceftriaxone, ciprofloxacin and several other AB. The most severe and hard-to-treat infections by enteric pathogens are caused by carbapenem-resistant (CRE) bacteria, which have become resistant to nearly all AB available today. In 2013, the US Department of Health and Human Services, Centers for Disease Control and Prevention (CDC), estimated that microorganisms from food and animals caused 1 in 5 resistant infections in people.
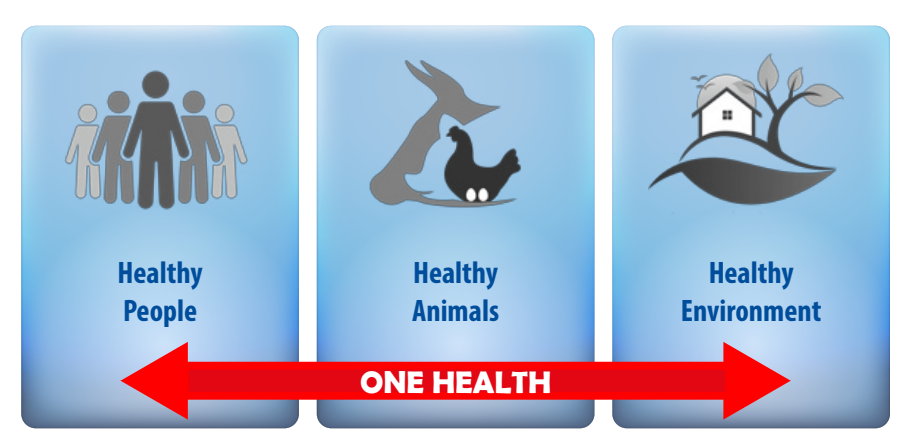
To combat the threat of infectious disease spreading between animals and people, the World Health Organization (WHO) recommended a holistic and multifactorial approach called “One Health”. One Health consists of a collaborative, multisectoral and transdisciplinary methodology, implemented on a local, national, and global scale to achieve the best outcomes for people, animals, plants and the environment. Figure 1 shows the connection between human, animal and environmental health of this strategy, which is widely supported and considered necessary for controlling the spread of ABR and ensuring the future of safe and sustainable animal food production.
Antimicrobial Resistance
Antimicrobial is a term used to describe medications that work against various microorganisms such as bacteria, viruses, fungi and parasites. Antimicrobial resistance (AMR) is a broader term used to describe resistance to medications against bacteria and other microorganisms such as fungi, parasites and viruses. Therefore, ABR is a component of AMR, regularly used in scientific and popular literature to refer to the emergent and growing threat of diverse resistant microorganisms besides bacteria.
Arrival of Antibiotic-Free Production
Since the late 1960s, the use of AB in food-producing animals has been the focus of a fierce debate. Sweden banned the use of growth-promoting AB in 1986, and Denmark banned the use of avoparcin and virginiamycin in 1995 and 1998, respectively. Other countries, or their poultry industries, introduced
voluntary bans around or after the year 2000. By 2006, the European Union (EU) had banned the use of all AB growth promoters in all member states. These bans were mandated based on a “Precautionary Principal” that affirms that when the health of humans and the environment is at stake, it may not be necessary to wait for scientific certainty to take protective action. Despite no clear demonstrated causal relationship between animal production and specific adverse public health outcomes, there has been a progressively strong movement supported by scientists, interest groups and politicians calling for the elimination or more strict restrictions for the use of AB in animal production. This concern results from the theoretical risk of rapid dissemination of resistant bacteria through the agricultural environment (soil and water) and food products, potentially causing untreatable illnesses in people handling or consuming these products. People also worry that poultry treated with AB could have residues in chicken, turkey and table eggs. These concerns have been accelerated in the modern information age in which consumers seek more transparency in how food- producing animals are raised. It is important to note that legislation in many countries, such as the USA and the EU, requires extensive monitoring of meat products to ensure that AB residues are not present in the food that reaches the marketplace.
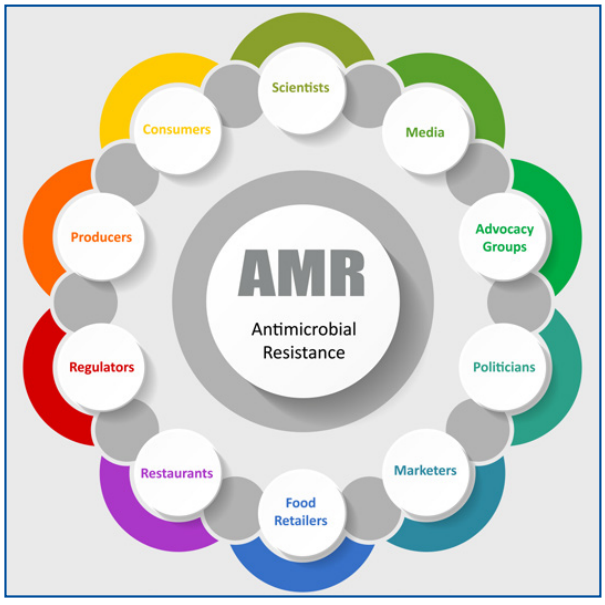
There is an increasing discussion about the potential dangers of animal food products contaminated with “superbugs” that could cause severe illnesses. Although there has been limited evidence, recent documented episodes of foodborne disease attributed to poultry products have increased unfavorable perceptions and decreased customer confidence. Consequently, all these issues have captured the attention of politicians, marketers, retailers and restaurants that are well attuned to customer demands and business opportunities.
Regulatory agencies have set new restrictions and requirements, which continue to be updated and modified to ensure food safety. Figure 2 shows the main groups involved in the discussion and evolution of public opinion and perception.
There is rising interest in alternative products, which has created a thriving new industry and the availability of numerous products with various purported beneficial attributes that can be used in animals of all ages. Research and development of alternative products (immune modulators, phytochemicals, probiotics, prebiotics, normal gut flora, yeast extracts, etc.) to reduce the need for and use of AB in food animals have demonstrated some benefits. Although results vary, and further research is needed to maximize their benefits under different field conditions, alternative products are being used alone, or most commonly in combinations, by producers worldwide as part of comprehensive health management programs.
Animal agriculture in general, and specifically poultry producers, have responded to public health concerns and consumer demands by implementing prudent guidelines for the judicious use of AB and production strategies that eliminate or minimize the need for them. Whether or not these changes in animal food production will reduce AMR risk and impact on the human population remains to be demonstrated. However, a new era characterized by significant AB usage reduction and/or antibiotic-free (ABF) poultry production has arrived. Today there is an even stronger commitment by producers and poultry professionals to protect public health and promote transparency and communication with customers.
Reductions in Antibiotic Use in the USA and UK
The National Chicken Council (NCC) has been the USA broiler industry’s leading voice supporting the judicious use of AB and veterinary oversight. In a statement released in 2015, the NCC affirmed that preserving AB effectiveness, both in humans and animals, is a responsibility of the producers. In addition, they conveyed their support of the Food and Drug Administration’s (FDA) Guidances #209 and #2013, and the Veterinary Food Directive (VFD) regulating the use of AB in animal feed. FDA guidance for industry (GFI) #2013, enacted in 2016, led to eliminating all medically important AB for humans in feed or drinking water for food-producing animals that previously could be purchased by producers over the counter and without a prescription and eliminated their use for growth promotion. Today, these medications can only be used for therapeutic purposes and under veterinary oversight. Before these regulations, the use of two classes of AB considered critically necessary for human medicine (fluoroquinolones and cephalosporins) had already been removed from chicken meat production. Table 1 shows a list and ranking by the USDA/ FDA of some AB used in poultry and their importance to human medicine. According to the FDA’s 2017 Summary Report, after implementing these policies, sales of medically essential antibacterial medications for chicken meat production decreased 43% from the previous year. Only 5% of AB sold for food-producing animals were intended for chickens. This report mentioned that ionophore medications used for coccidiosis control were not considered medically important, as they do not pose significant cross-resistance risks for public health and are not typically used for human medicine. In 2019, a report sponsored by the FDA and US Poultry and Egg Association (USPEA) showed that over a 5-year period ending in 2017, the broiler industry drastically reduced the use of medically important AB as a result of FDA regulations, greater focus on disease prevention (hygiene, nutrition and vaccination), and improved tracking of AB usage. The data was provided voluntarily by producers and represented over 7.5 billion chickens (nearly 90% of the USA annual production). Some of the key changes reported were as follows:
- Chicks receiving AB at hatch decreased from 93% to 17%.
- The use of gentamycin in the hatchery decreased by approximately 74%.
- In-feed use of medically important AB decreased by 95%.
- Water-soluble, medically necessary AB decreased significantly.
Table 1. Ranking of some AB used in poultry according to their importance in human medicine.
Important |
Highly Important |
Critically Important |
Cephalosporins, 1st and 2nd generation |
Aminoglycosides |
Cephalosporins, 3rd generation |
Cephamycin |
Carbapenems |
Fluoroquinolones |
Monobactams |
Cephalosporins, 4th generation |
Macrolides |
Quinolones |
Chloramphenicol |
Sulfas/trimethoprim |
Glycopeptides |
||
Metronidazole |
||
Penicillin (different types) |
||
Streptogramins |
||
Tetracyclines |
In the UK, the British Poultry Council’s (BPC) 2018 report showed:
- An 82% reduction in the use of AB since 2012.
- The poultry meat sector alone achieved a 60.44% reduction during the preceding 6-year period.
- Fluoroquinolones use (considered a critically important human AB) decreased by 91%.
- The industry used 9.72% of the total AB approved for food-producing animals compared with 21% in 2012 despite increasing production output by 10%; this is in a country where about half of all meat eaten is poultry.
Furthermore, the British poultry meat sector has:
- Stopped prophylactic use of AB.
- Stopped use of colistin.
- Only uses fluoroquinolones and macrolides as a last resort and following with specific rules to follow.
- Banned the use of third and fourth-generation cephalosporins.
- Restricted the use of AB classified as the highest priority critically important by the WHO.
Given that these changes occurred without regulatory action, the BPC attributed this progress to the industry’s commitment to responsible AB stewardship through innovative approaches. The BPC’s approach was based on a policy of reducing, refining and replacing. The BPC’s report suggests these reductions were achieved using the following strategies:
- Data collection
- Rapid on-farm diagnostics
- Sharing best practices
- Understanding patterns of resistance
- Looking at alternative strategies
The reduced use of AB reported in the USA and UK, and the implementation of similar AB reduction programs in many other countries, assure that the industry can protect bird health and welfare while safeguarding human health. These results demonstrate a different perspective that can help improve public perceptions about the industry. Poultry production organizations remain committed to continued stewardship efforts and have consistently stated that total elimination of AB use is unethical, as it prevents the fulfillment of a responsibility to alleviate animal pain and suffering when necessary.
Stewardship, Role of Veterinarians and Poultry Managers
Veterinary and health organizations around the world agree on the importance of the judicious use of AB. These organizations continue to advocate effective use through carefully planned and executed practices in areas such as vaccination, health monitoring, diagnostics, biosecurity, husbandry and management programs.
In 2018 the American Veterinary Medical Association (AVMA) and the American Association of Avian Pathologists (AAAP) updated their guidelines for the judicious use of AB. These organizations defined antimicrobial stewardship (which includes the use of AB) as actions performed by veterinarians, individually and as a profession, to preserve the effectiveness and availability of these products through supervision and responsible use while safeguarding animal, public, and environmental health. According to the AAAP, stewardship for poultry veterinarians involves the following:
- Taking responsibility for maintaining health and welfare, and implementing preventive and management strategies.
- Using an evidence-based approach in making decisions regarding the use of antimicrobial medications.
- Using antimicrobials judiciously, sparingly, and with continual evaluation of the outcomes.
- Protecting poultry health and ensuring safe, affordable food to consumers.
In addition, the AAAP established stewardship principles. A summary of these is presented below:
- Commitment
- Engaging all production personnel.
- Developing plans incorporating accountability for disease prevention, control, and treatment.
- Advocating a system of care to prevent, treat and control diseases
- Promoting improvements in farm management, biosecurity, and vaccination to minimize the need for antimicrobials.
- Providing alternative strategies.
- Selecting and using antimicrobial medications judiciously
- Using an evidence-based approach for making a diagnosis.
- Determining the need and selecting an appropriate antimicrobial therapy.
- Evaluating antimicrobial medication use
- Continuous evaluation of antimicrobial medication prescribing practices.
- Supporting analysis and sharing of antimicrobial medication use data.
- Educating and building expertise
- Making resources available and encouraging education in antimicrobial stewardship, management, biosecurity and production practices.
- Supporting research on antimicrobial medication use and resistance.
According to FDA regulations, medically important AB can only be used to prevent, control and treat specific diseases. When these are given through the water or feed, they require a veterinary prescription or feed directive. To prescribe such AB, veterinarians must have a valid client-patient-relationship (VCPR). This relationship is the basis for the interaction between veterinarians, their clients and their patients, and has the following requirements:
- The veterinarian has assumed the responsibility for making clinical judgments regarding the patient’s health, and the client has agreed to follow the veterinarian’s instructions.
- The veterinarian has sufficient knowledge of the patient to initiate a general or preliminary diagnosis of the patient’s medical condition. This means that the veterinarian is personally acquainted with the patient’s keeping and care by virtue of a timely examination, or medically appropriate and timely visits by the veterinarian to the operation where the patient is managed.
- The veterinarian is readily available for follow-up evaluation or has arranged for veterinary emergency coverage and continuing care and treatment.
- The veterinarian provides oversight of treatment, compliance and outcome.
- Patient records are maintained.
Poultry veterinarians work with flocks of domestic poultry rather than individual animals. Therefore, “sufficient knowledge of the patient” means that poultry veterinarians must:
- Conduct medically appropriate and timely visits to the facility where poultry flocks are housed.
- Examine representative patients/birds and review medical records and laboratory or diagnostic procedure records.
- Consult with those individuals providing care to the birds regarding ongoing health management programs.
Recently, the AVMA’s Committee on Antimicrobials published concise definitions of antimicrobial uses to prevent, control and treat disease (JAVMA/April 1, 2019/ Vol. 254, No.7). These definitions were necessary to avoid confusion and to help veterinarians clearly communicate their reasoning when prescribing or recommending the use of antimicrobials. These designations are the basis of the current AVMA policy for prevention (prophylaxis), control (metaphylaxis), and treatment of disease in animals individually and on a population basis.
For each specific AB or antimicrobial used in food-producing animals, regulatory requirements establish a particular time of “no AB use”, also known as the “withdrawal period” before harvest. This period is the time required for the bird to eliminate these products from their systems, so no residues are present when animals are processed for human consumption. Therefore, veterinarians assist producers by ensuring that antimicrobials are administered in compliance with withdrawal times established for specific products.
Additional oversight (verification of compliance) is conducted by regulatory agencies involved in the monitoring, detecting and controlling residues in poultry products. Interestingly, many retailers and consumers in the USA are not aware that due to regulatory requirements, all chicken meat sold contains no AB or residues, and that all flocks are raised under health programs designed by licensed poultry veterinarians.
In Europe and other parts of the world, there is a similar legislation requiring that all AB are prescribed by a veterinarian who must follow specific guidelines. They may only prescribe AB for poultry under their care, and there are specific guidelines for when the poultry are placed in the care of a named veterinarian. Additionally, industry bodies such as the Responsible Use of Medicines in Agriculture Alliance (RUMA) in the UK have been in action for many years. RUMA is an associate member of the European Platform for Responsible Use of Medicines in Animals (EPRUMA). European veterinary organizations like the British Veterinary Poultry Association (BVPA) also have specific guidelines for AB use that are regularly updated, with the last update published in 2018.
Despite all of the on-going industry and veterinary efforts to prevent poultry diseases, some flocks become sick, and AB treatment is a necessary and justifiable option for the poultry veterinarian. A growing trend in the food supply chain (i.e. food retailers and restaurants) is the requirement for poultry meat or eggs produced with no AB ever (NAE), and in general, these products command a premium price. As a result, when a flock becomes sick and must be treated with AB for health and welfare reasons, its products cannot be labeled as NAE and enter that market. Consequently, this creates an economic dilemma for the producers when a flock becomes sick and must be treated with AB since its meat or eggs have to be diverted to other acceptable market sectors. This meat or eggs is typically sold at a lower price - despite complying with required withdrawal periods and freedom of residues before entering the food chain.
Veterinarians take an oath guiding and affirming their use of scientific knowledge and skills for the benefit of society by protecting animal health and welfare, the prevention and relief of animal suffering, the conservation of animal resources, the promotion of public health and the advancement of medical knowledge. Therefore, veterinarians must be able to prescribe proper treatment plans for animal health and animal welfare, including the use of an AB when this option is justified, to conform with their professional commitment and ethical obligation. Antimicrobial stewardship is a shared responsibility for veterinarians, regulatory agencies, and poultry production managers. Commitment to the use of antimicrobials in a judicious manner and continuous collaboration among all stakeholders is essential to ensure poultry welfare and the industry’s future.
Challenges and Opportunities
In the USA, ABF chicken meat production started as a small-scale production system to satisfy the needs of niche or premium pricing markets. As the use of AB was identified and widely publicized as a potential risk factor in the development of AMR, ABF production increased and became a defining industry trend impacting marketing strategies, consumer preferences (growing perception that ABF chicken is better) and regulatory initiatives to phase out medically important AB from animal agriculture.
Chickens raised in the USA without AB of any type consumed 40% of the broiler feed produced in 2017, according to Rennier and Associates Inc. of Columbia, MO., (a firm that conducts surveys and trend analysis of the poultry industry). This amount was double the quantity of feed for NAE programs produced in 2016 and a 10-fold increase from the 4% level in 2013. Today, it is estimated that NAE production is > 50% of USA broiler production, and several integrated broiler companies are now committed to this system. There are four distinct programs as described by Rennier and Associates:
- Conventional - Potential use of all FDA-approved poultry medications.
- Reduced Use - Programs avoiding medically important AB.
- Ionophores Only - Following the World Organization for Animal Health’s (OIE) guidelines allowing the use of ionophore coccidiostats.
- NAE - No AB of any kind during the flocks’ life, even if the need is therapeutic. This includes no use of ionophore coccidiostats.
NAE or “Raised without AB” (RWA) are product designations used by producers in the USA to label poultry products that have been successfully raised without any AB, whether classified as essential in human medicine or not. Flocks that have been therapeutically treated due to disease diagnosis are not eligible for NAE or RWA designation.
The Pew Charitable Trust and School Food Focus organizations developed a Certified Responsible AB Use (CRAU) designation for institutional buyers of poultry products. In 2015, the United States Department of Agriculture (USDA), Agricultural Marketing Service (AMS) approved CRAU broiler companies through audits performed by a USDA Process Verified Program (PVP) or the USDA Quality System Assessment Program (QSA). Producers complying with the CRAU designation are prohibited from using AB with analogs in human medicine routinely or without clear justification. The use of products with analogs in human medicine must be rare, well documented and prescribed by a licensed veterinarian. CRAU standards have no further restrictions for products with no analogs with veterinary medicine (such as ionophores); they are considered little or no risk to public health. The CRAU designation is recognized as another initiative to reduce AB use while protecting human and animal health and allowing the marketing of wholesome and affordable products.
At the moment, European countries (EU/ EEA)*1 classify ionophore coccidiostats as feed additives, despite pressure from some interest groups to label them as AB. Ionophores are not considered medically important AB by the WHO or OIE. Producers in the EU have maintained that coccidiosis is an endemic disease, and therefore, ionophores are a safe and effective option to ensure welfare standards and sustainable broiler production. Although some European countries have a significant proportion of poultry grown as ABF, most EU countries’ producers strive towards responsible use rather than NAE-like systems. With this approach, they have managed to produce many flocks without AB (not including ionophores) and low levels of AB use in poultry production.
*1 The EEA includes EU countries plus Iceland, Liechtenstein and Norway
The shift to ABF production has come with some challenges. Several reports suggest that raising chickens without AB could reduce performance compared with traditional programs (i.e. unrestricted access to judicious use of all approved medications). This is possibly due to higher mortality because of neonatal infections and/or a higher incidence of intestinal health problems such as coccidiosis and necrotic enteritis. Also, concerns about negative impacts on animal welfare have been raised. Discernibly, there has been a learning curve since the start of ABF production, and broiler operations worldwide continue to identify key factors, upgrade their practices, develop new disease prevention strategies and make gradual improvements.
More recent studies and field experiences have shown that it is possible to achieve similar performance and cost-efficiency as traditional systems in the absence of AB. During the last few years there has been an abundance of scientific and industry publications on strategies to make ABF production successful. Achieving this goal depends on a comprehensive review and improvements in the following areas:
- Management practices (breeders, hatchery, and broilers)
- Chick quality
- House environmental controls
- Litter management and treatments
- Water quality, diet composition and feeding practices
- Gastrointestinal development and health (including gut microbiota)
- Biosecurity, downtime between flocks, cleaning and disinfection practices
- Control of coccidiosis and necrotic enteritis
- Prevention of immunosuppressive conditions
- Use of alternative products (feed additives to promote gut health)
- Monitoring of flock behavior, health and performance (transition times)
- Pre-harvest food safety
The broiler industry continues to thrive and, as the world population grows, the demand for safe, affordable and nutritious animal protein continues to rise, particularly in developing economies around the world.
Poultry products continue the trend to become the protein of choice on a global basis. Consequently, poultry professionals and production managers will likely be confronted with additional customer and regulatory requirements. The industry must continue to evolve through innovation, communication, transparency and technology.
Conclusions
Following this introductory review, Aviagen® will produce and distribute a series of technical updates to address key broiler management practices applicable when electing to produce broiler meat production under ABF conditions. Future technical updates will be derived from reviews of scientific and technical publications, consultation with renowned poultry specialists, and field experiences of Aviagen global technical teams.









