



Avian infectious bronchitis virus: infection, evolution, and immunity
Dr. Tahseen Aziz shares new insight into infectious bronchitis virus (IBV), a Gammacoronavirus in the family Coronaviridae, which also includes three other genera: Alphacoronavirus, Betacoronavirus, and Deltacoronavirus.Coronaviruses are very host specific; alpha- and betacoronaviruses only infect mammals, gammacoronaviruses only infect poultry, and deltacoronaviruses have a wider host range and include wild birds and some mammals. Coronaviruses are enveloped, single-stranded RNA viruses. The virions (complete, infective virus particles) are spherical with a diameter of approximately 125 nanometer (nm). Coronaviruses have distinct, club shaped, spike structures on the surface, thus the name corona (in Latin, corona = crown). They have an unusually large genome, which for IBV is ~27,600 nucleotides. The envelope that surrounds the virus is composed of two layers of lipid that contain viral proteins. These viral proteins are necessary for infectivity of the virus, and thus when the envelop is destroyed, the virus becomes non-infective. The lipid envelope is readily damaged by desiccation, heat, and detergents, and therefore the virus has limited survivability outside the cell and is relatively easy to inactivate by cleaning and disinfection.
Major proteins of the virus
The infectious bronchitis virus genome encodes four major structural proteins: spike (S), membrane (M), envelope (E), and nucleocapsid (N). The S, M, and E proteins are associated with the virus envelope.
Spike protein
This is a glycoprotein that makes up the distinctive club-shaped spike structures on the surface of the virus. The protein mediates the virus attachment to and entry into host cell. It has two subunits: the S1 subunit (approx. 500-550 amino acids) is responsible for recognition and binding of the virus to the host cell receptor, and the S2 subunit (630 amino acids) is responsible for fusion of the virus to the cell membrane, allowing the viral genome to enter host cells. Among all structural proteins of IBV, spike protein is the main antigenic component that is responsible for inducing neutralizing antibodies and protective immunity against the virus. The S1 subunit of the protein contains several epitopes (antigenic determinants) for virus-neutralizing, serotype-specific antibodies. Three epitopes have also been identified in the S2 subunit, but the virus-neutralizing activity of antibodies induced by these epitopes is uncertain.
Membrane protein
This glycoprotein is the most abundant structural protein in the virus virion. It is thought that the protein maintains the shape of the virus.
Envelope protein
The envelope protein is present in small quantities within the virus. The main function of this protein is to facilitate assembly and release of the virus from the host cell.
Nucleocapsid protein
This protein is formed during virion assembly when it binds to viral RNA. It is the most abundant protein of coronaviruses. The protein is a major immunogen that incites the cellular immune response.
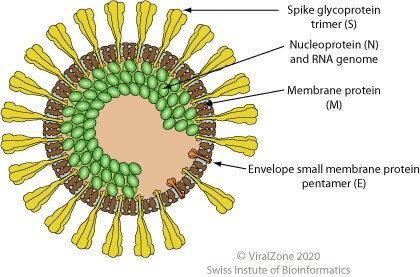
© ViralZone, Swiss Institute of Bioinformatics
Initiation of infection
To successfully initiate infection, the virus must overcome the cell membrane barrier. This is achieved by fusion of the virus to the cell membrane. Upon attachment of the virus to cell receptors, a process mediated by the S1 unit of the S protein, the S2 unit of the S protein fuses with the cell membrane of the host cell, and the viral RNA enters the cell. The virus replicates and is assembled in the cytoplasm of the host cell. It is then released and acquires the lipid envelope by budding through the cell membrane.
Virus genotypes, strains, serotypes
A genomic classification scheme based on sequencing and analysis of the whole S1 gene has been proposed for IBV. In this this scheme, IBV was grouped into six, genetically divergent genotypes (GI, GII, GIII, GIV, GV, GVI) with 32 lineages. Twenty-seven lineages were placed in genotype I. Each of the remaining six genotypes contains one lineage. Some of these lineages are distributed in several continents, countries, or regions, while others are indigenous to certain geographical areas. Each lineage comprises different viruses, each referred to as strain and given a specific designation (e.g. GA08, Arkansas, QX, Italy02, etc.). Thus, according to this genotyping scheme, the term strain refers to the names of viruses within a lineage in a genotype.
Gene sequencing and phylogenetic analysis of IBV strains suggest that different strains may have been derived from the same ancestor (Massachusetts IBV) by gene mutation and genetic recombination due to immunological pressure, frequent mixed infection, and widespread use of live vaccines. The degree of genomic relatedness varies between strains.
There is no serotyping classification system for IBV, but cross-neutralization assays in specific-pathogen free chicken eggs are used to determine the immunologic cross reactivity between strains. Generally, serotype groups correspond to genotype groups in the classification system.
Evolution of the virus
Infectious bronchitis virus is a continually evolving virus. New variants of the virus continue to emerge globally due to mutation in the virus genome. Spontaneous errors (nucleotide substitution, deletions, insertions, and recombination) during virus replication result in virus evolution and emerging of new variants. Mutation in the gene coding for a specific viral protein can result in changes in the amino acid composition of the protein. IBV variants are defined by changes in the amino acid composition of the S1 protein due to mutation in the gene coding for this protein, and the emerging of new variants can result from only a few changes in the amino acid composition. Comparison of the entire S1 gene has been commonly used to determine IBV strains relatedness and for elucidation of IBV evolution. The S2 subunit gene is more conserved than the S1 subunit gene and has a high level of nucleotide identity between variants. It is possible that changes in other viral proteins due to genome mutation may influence the virulence of the virus.
Immunity
As mentioned previously, the S1 protein is the main antigenic component of the virus; it contains the epitopes (short chains of amino acids within the protein) responsible for inducing neutralizing antibodies and protective immunity. The epitopes of different strains may vary in amino acid composition, which can change the three-dimensional shape of the protein, and thus immunity induced by one strain does not provide sufficient protection against other strains. The ability of IBV to evolve and change creates a challenge in protecting flocks against the disease by using the commercially available vaccines, as vaccines targeting individual IBV strains provide poor cross-protection. Some strains are more closely genomically and antigenically related (Mass and Conn) than others (GA98 and Ark) and so they tend to offer more cross-protection against each other.
Local immunity vs systemic immunity
Respiratory (nasal passages, trachea) and conjunctival epithelium is the initial site of entry and replication of IBV, thus local mucosal immunity in the respiratory tract plays a major role in protecting the bird. Local antibodies (mainly IgA) neutralize the virus and inhibit its attachment to the mucosal epithelial cells. Live-attenuated vaccines administered by spray stimulate local and cell-mediated immunity. The Harderian and lacrimal glands produce local IgA while activated CD8+ and CD4+ lymphocytes play a role in clearing the infection of the respiratory tract.
The role of circulating antibodies in protecting against respiratory infection is controversial. Several studies have shown that circulating antibody titers do not correlate with protection of the respiratory tract. However, high levels of circulating antibodies in laying hens are extremely important to protect against infection of the reproductive tract, drop in egg production drop, and deterioration of egg-shell quality. Circulating antibodies (mostly IgG) induced by vaccines limit the spread of the virus from the respiratory tract to other susceptible organs, including the kidneys and reproductive tract.
Vaccination
Infectious bronchitis is a disease that requires vaccination to be part of the control strategy. Live-attenuated and inactivated vaccines are available. Inactivated vaccines contain adjuvant to induce strong, long-lasting immunity, but do not induce a strong cellular immune response. In broilers, only live vaccines are used due to the short life span of the bird. In long-lived birds like layers and breeders, vaccination programs include either live vaccines only or a combination of live and inactivated vaccines. With a properly designed vaccination program, proper handling of the vaccines, and administration of full doses, live vaccines alone potentially achieve adequate field protection similar to live/inactivated vaccine programs. Live vaccines are usually applied every 3-6 weeks starting by 2 weeks of age with each successive vaccination building on the previous by either diversification of serotypes or increasing immunogenicity using more “active” vaccines or application methods. Live boosters during lay should also be administered every 6-8 weeks in all-live programs to maintain local immunity during production. In a live/inactivated program, live vaccines are given first to protect the pullet as well as prime the immune system for optimal response to the inactivated vaccination(s). The combination of cellular (live) and strong humoral (inactivated) immunity best prepares the hen for field challenges through peak production and beyond.
The continual emergence of new field variants is a persistent problem to the poultry industry. No single vaccine can provide full protection against different stains/variants. A vaccine virus with S1 protein amino acid sequence homologous to that of a field virus may give full protection, but it is impossible to develop a live vaccine for all the different strains/variants. It has been shown, however, that certain vaccines may provide better partial protection than others against a particular field virus. Under field conditions, a combination of two or more types of IBV vaccines are often used to maximize cross-protection. Cross-protection of vaccines against a field virus is best determined by testing in chickens. In commercial poultry flocks, a trial-and-error approach may be used to determine the best vaccine or vaccine combination against field virus endemic in the region.
The cross-protection of heterologous vaccine virus may be due to neutralizing antibodies binding to conserved epitopes in the S1 protein. As mentioned earlier, there are several epitopes in the S1 protein. Mutation in the S1 gene may result in changing the amino acid sequence of only some epitopes. The remaining conserved epitopes can still incite homologous antibodies, thus providing protective immunity. Additionally, the S2 protein, which is more conserved that the S1 protein, also has epitopes that may play a role in inciting protective antibodies.
Maternal antibodies (passive immunity) and protection
Maternal antibodies of IBV may provide only short-term protection of newly hatched against infection. Antibodies (IgG) pass from vaccinated hens to the progeny via the yolk and are present in the serum and respiratory mucus of chicks. In one study, chicks hatched with high levels of maternally derived antibodies were found to have excellent protection against IBV challenge at 1 day of age but not at 7 days. This protection was significantly correlated with levels of local respiratory antibody and not with serum antibody. Interestingly in another study, vaccination of 1-day-old chicks with live vaccine induced a rapid decline in maternally antibodies due to binding and partial neutralization of vaccine viruses. Because of the short-lived maternal antibodies, newly hatched chicks are sprayed with live-attenuated vaccine in the hatchery to induce an active immune response. Broilers that are processed over 50 days of age are sometimes boosted with another spray vaccination between 16 and 18 days of age.
The concept of trachea health monitoring
Infectious bronchitis virus causes severe damage to the trachea, and it predisposes chickens to secondary bacterial infections, particularly with E. coli. Histopathologic profiling of tracheas of chickens in a commercial flock at certain ages may provide information about the protection efficacy of IBV vaccines used in the flock. Of course, keeping in mind that tracheitis is can be caused by other respiratory pathogens. A scoring system based on the histopathologic changes in the tracheas can be developed to evaluate the trachea health. This is a new concept that may need further exploration.
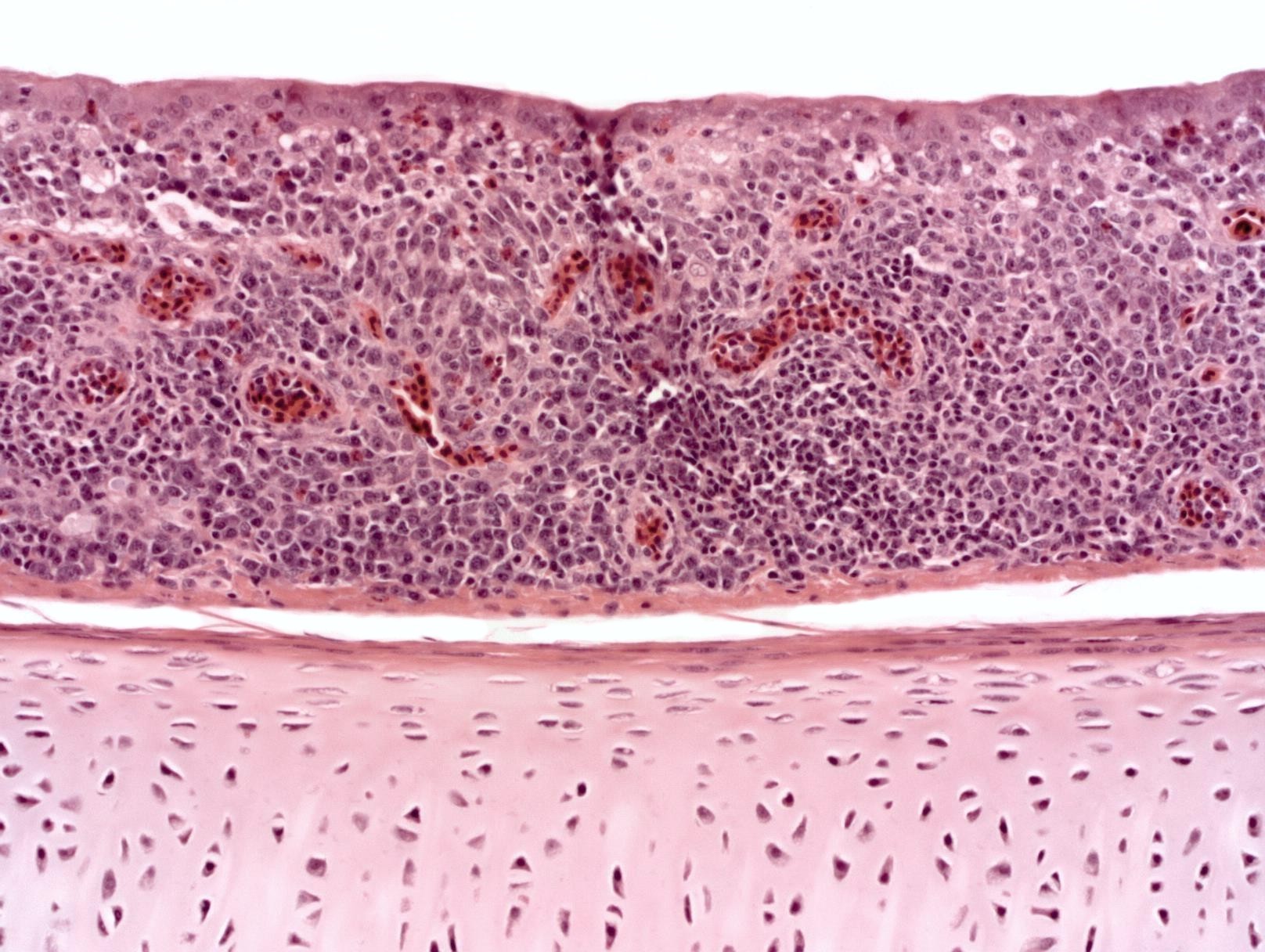
The trachea was positive for infectious bronchitis virus by PCR test.
Thoughts from the author
The disease name infectious bronchitis is really a misnomer. Lesions in the pulmonary bronchial tree (bronchitis lesion) are usually absent or minimal. Thus, the name infectious bronchitis does not reflect the tissue pathogenesis of the virus. Tracheitis is the consistent lesion caused by IBV in chickens. Airsacculitis may be severe in infection with some variants. The name “avian coronavirus tracheitis” may be more accurate to reflect the marked pathogenicity of the virus for the trachea. From my experience as an avian pathologist and diagnostician in a veterinary diagnostic laboratory, diagnosis of infectious bronchitis is best achieved by histopathologic examination of tracheas and by PCR testing of tracheas for IBV. Histopathology and PCR results should always be interpreted in conjunction with each other to establish a diagnosis of infectious bronchitis.
Acknowledgement
The author would like to thank Dr. Brian Jordan Dr. Kalen Cookson for their input and editorial assistance.









