



Avian Influenza in Poultry
In 1878 a respiratory disease causing high mortality in poultry was first described as Fowl Plague. In 1955 an influenza virus was isolated as the cause of Fowl Plague or avian influenza. This article provides a few fact about Avian Influenza and provides links to more detailed information on this destructive disease.Detailed information on |
A clinical sign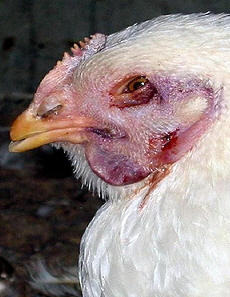 |
A few facts about Avian Influenza
Introduction
Avian Influenza (AI) is a contagious viral infection which can affect all
species of birds. In intensive poultry rearing systems young fattening
turkeys and laying hens are usually the most affected species.
Free-living birds may carry influenza viruses without becoming ill due to
a natural resistance. It is known that wild waterfowl present a natural
reservoir for these viruses and can be responsible for the primary
introduction of infection into domestic poultry.
Causative agent
The virus causing avian influenza is an Influenzavirus A virus of the
family Orthomyxoviridae. Several virus subtypes exist, which are divided
on the bases of the antigenic relationships in the virus glycoproteins
haemoagglutinin (H) and neuraminidase (N). At present 15 H subtypes
have been recognised (H1-H15) and nine neuraminidase subtypes (N1-
N9).
Influenza A viruses infecting poultry can also be divided on the basis of
their pathogenicity (ability to cause disease).
The very virulent viruses cause highly pathogenic avian influenza (HPAI)
with mortality in poultry as high as 100%. In the whole world there have
been only 19 reported primary isolates of such viruses from domestic
poultry since 1959. A severe epidemic occurred in Italy in 1999/2000
causing 413 outbreaks with 16 Million birds affected.
Other AI viruses cause a much milder disease (low pathogenic avian
influenza, LPAI). Clinical signs are much less evident or even absent and
mortality is much lower.
Sometimes secondary infections or environmental conditions may cause
exacerbation of LPAI infections leading to more serious disease.
Evidence suggests that certain avian influenza virus subtypes of low
pathogenicity may, after circulation for some time in a poultry
population, mutate into highly pathogenic virus strains.
To date only viruses of H5 and H7 subtype have been shown to cause
HPAI in susceptible species, but not all H5 and H7 viruses are highly
pathogenic.
Clinical symptoms
The main symptoms of HPAI in poultry are depression, loss of appetite,
cessation of egg laying, nervous signs, swelling and blue discoloration of
combs and wattles due to disturbance of blood circulation, coughing,
sneezing and diarrhoea. Sudden death can occur without any previous
signs. The mortality rate may reach up to 100% depending on the species,
their age, the virus type involved and environmental factors like
concurrent bacterial infections.
Clinical signs of LPAI consist primarily of mild respiratory disease,
depression and drop in egg production in laying birds.
The incubation periods for these viruses range from as short as a few
hours to 3 days in individual birds and up to 14 days to spread throughout
a flock.
Transmission and spread
All the available evidence suggests that the most common primary
introduction of AI viruses into an area is by wild birds, usually waterfowl,
but gulls and shorebirds have also been implicated. Direct contact
between wild bird and poultry is not always necessary for introduction of
virus into poultry farms, as infected waterfowl may spread AI viruses by
infective faeces into an area and these may then be introduced to poultry
farms by a variety of mechanisms that may transfer the virus
mechanically. If contaminated with influenza viruses, surface water used
as drinking water may also be a source of infection. Poultry kept in free
range or poultry which have access to surface water are at specific risk.
AI is transmitted within a farm by direct contact of infected animals with
healthy animals, or indirect contacts with contaminated equipment or
farm staff.
Spread of AI viruses from farm to farm is mainly by mechanical transfer
of infective faeces, in which virus may be present at high concentrations
and may survive for considerable periods. Shared water or food may also
become contaminated.
However, man is a very important cause of secondary spread of AI for
domestic poultry. Caretakers, farmers, workers, trucks and drivers
visiting farms, moving birds or delivering food have caused the spread of
AI virus both on to and within farms.
Legislation and basic disease control measures
The O.I.E (Office International des Epizooties, the World Organisation
for Animal Health) has classified HPAI as a "list A" disease, signifying a
rapidly spreading animal disease of major economic importance, such as
Foot and mouth disease or classical swine fever.
EU legislation to control avian influenza is laid down in Council
Directive 92/40/EEC. All suspected cases of AI must be investigated and
appropriate measures taken in case of confirmation of HPAI. To limit the
spread, infected poultry must be killed in a humane way and disposed off
safely. Feedingstuffs, contaminated equipment and manure must be
destroyed or treated to inactivate the virus.
To prevent further spread of disease the veterinary authorities are
required to immediately put in place movement restrictions on the
affected holdings and on all farms in a radius of at least 10-km around
these holdings, the so called surveillance zone. If necessary, stamping-out
measures can also be extended to poultry farms in the vicinity of or which
have had dangerous contacts with infected farms.
In accordance with Community legislation, all Member States have AI
contingency plans in place to ensure that the most appropriate measures
are immediately implemented.
At farm level preventive hygienic measures such as cleaning and
disinfection are crucial. Disease awareness amongst farmers and cooperation
by all people in the poultry sector must ensure that the strictest
biosecurity measures are applied to prevent disease spread.
Vaccination
The existence of a large number of virus subtypes together with the
known variation of different strains within a subtype pose serious
problems when selecting strains to produce influenza vaccines and to use
vaccination as a routine tool for disease prevention.
In accordance with Directive 92/40/EEC, vaccination against AI may be
used to supplement the control measures carried out after confirmation of
disease. Birds vaccinated against the HA subtype corresponding to the
one which is circulating are protected against the worst effects of AI.
The decision to introduce vaccination may be taken by the Member State
concerned, with or without prior approval by the Commission. Such steps
must be accompanied by further disease control measures, including trade
restrictions, in accordance with the Standing Committee procedures(1).
Following confirmation of LPAI, vaccination against AI is currently
being applied in some regions of Italy, pursuant to Commission Decision
2002/975/EC(2). This vaccination strategy developed and applied in Italy
makes use of a heterologous vaccine, allowing discrimination between
vaccinated and infected poultry(3). This strategy was adopted for the first
time in the world in 2001 by Commission Decision 2001/847/EC(4) and
allowed certain trade restrictions on the meat of vaccinated poultry to be
lifted.
However, the immunity induced by vaccination may not be sufficiently
rapid to stop farm-to-farm spread of HPAI. Furthermore, emergency
vaccination is also hindered by practical difficulties related to the
administration of the vaccine (each single bird must be injected).
|
1 Certain specific disease control measures may be taken by the Commission, following a debate in the Standing Committee for the Food Chain and Animal Health, which includes representatives of all Member States. 2 Commission Decision 2002/975/EC of 12 December 2002 on introducing vaccination to supplement the measures to control infections with low pathogenic avian influenza in Italy and on specific movement control measures, Official Journal L 337, 13.12.2002, p 87. 3 The vaccine is prepared with a virus strain which has the same H type of the field virus circulating in the area, but with a different N type. The vaccinated birds are protected against the field virus but they produce antibodies against a different neuraminidase. A serological test may then allow discrimination. 4 Commission Decision 2001/847/EC amending for the third time Decision 2000/721/EC to modify the Italian avian influenza vaccination programme and current trade restrictions for fresh meat originating from vaccinated turkeys Official Journal L 315, 1.12.2001, p 61. |
Potential threat to human health
The human population all over the world is continuously affected by
epidemic waves of Influenza due to virus strains of human origin, causing
mainly respiratory infections which may be particularly serious in the
young and elderly people and in the immuno-depressed individuals.
Vaccines are available and their use is recommended for at-risk groups.
However, influenza viruses of avian origin may also be occasionally
responsible for disease in humans. In recent years three different subtypes
of avian influenza virus have been detected on five occasions in humans,
causing disease of varying degrees of seriousness. In all cases the avian
influenza viruses have shown a very limited tendency to spread in the
human population (the spread was self-limiting).
In 1996, a H7N7 virus of avian origin was isolated in England from the
eye of a woman with conjunctivitis who kept ducks. In March 1999 two
independent isolations of influenza virus subtype H9N2 were made from
girls aged one and four who recovered from flu-like illnesses in Hong
Kong. Subsequently, five isolations of H9N2 virus from humans on
mainland China in August 1998 were reported.
The most serious case occurred in May 1997 in Hong Kong. A virus of
H5N1 subtype was isolated from a young child who died in Hong Kong
and by December 1997 the same virus was confirmed by isolation to have
infected 18 people, six of whom died. There was evidence of very limited
human to human spread of this virus, but the efficiency of transmission
amongst humans was extremely low. In February, 2003 a new
transmission of avian influenza virus subtype H5N1 to humans was
reported in Hong Kong, infecting 4 people of one family, two of whom
died. In Europe several cases of avian influenza have been identified, but
they have never had any human consequences.
The exact mode of transmission of these viruses to humans is not known.
In this context, surveillance for influenza viruses in humans and animals
is relevant for both human and animal health. The disease requires a high
level of preparedness, both at national and at Community level. The
Commission has identified influenza as a priority area within the
Community Network for Communicable Diseases. A disease surveillance
network is in place under the decision 2119/98/EC (EISS: European
Influenza Surveillance Scheme) to monitor virological and clinical data
concerning influenza in 18 European countries. These activities are
carried out in collaboration with the other existing surveillance networks,
including those co-ordinated by WHO.
Further Reading
More articles on Avian Influenza can be found by Clicking Here
Source: www.avian-influenza.com - January 2005









