Cloned vaccines against Newcastle disease virus, heterologous protection against genotypes VII a XII
Given the different methods of poultry production in different countries and the presence of wild birds and their natural behaviour and migratory routes, it remains difficult to eradicate Newcastle disease and bring down its currently high rates of occurrence, as it is still endemic in most countries in Asia, the Middle East, Africa, Central America and South America.by Javier S. Corella of Hipra
Introduction
The appearance of very aggressive outbreaks of Newcastle disease is caused by velogenic strains with genotypes different from those present in the classic vaccines currently being used, resulting in doubts about the efficacy of classical live attenuated vaccines. Newcastle disease (ND) is caused by virulent forms of avian Paramyxovirus serotype 1 (APMV-1). Newcastle Disease virus (NDV) is classified into different classes and genotypes based on its genetic characteristics. Epidemiological control of Newcastle disease in broilers is possible (amongst other tools) thanks to the intensive use of prophylactic vaccination. In general, the most common vaccines in the world against this disease are based on two main genotypes of live lentogenic NDV (genotype I and II), characterised by low pathogenicity or apathogenicity. The genetic divergence between the genotypes of the vaccines and the circulating viruses raises questions about their efficacy.
An independent study by Jeon et al. demonstrated the efficacy (reduction of clinical signs and control of the spread of the virus) of a commercial killed vaccine containing the LaSota strain against velogenic genotype VII outbreaks in Korea.
However, there are not many studies to evaluate the protection produced by live vaccines with other types of strains such as cloned strains of LaSota, combined vaccines against Newcastle and infectious bronchitis, vaccination programs or the administration routes used.
HIPRAVIAR® CLON, vaccination program and route of administration against NDV genotype XII.
HIPRAVIAR® CLON and HIPRAVIAR® CLON/H120 vaccines are composed of a live attenuated LaSota cloned strain (CL/79) originating from the LaSota strain of the Newcastle Disease virus (NDV) with a titration >106.5 EID50. The use of cloned strains represents a technological breakthrough in these types of vaccines, since they make possible to have a completely uniform vaccine virus population, with specific viral characteristics, determined by the viral selection process. This vaccine has good commercial acceptance due to its high immunogenicity and low residual pathogenicity when compared with the LaSota parental strain, in addition there is also a reduction in the severity of the post-vaccine reactions.
In a study conducted on the experimental farms of the Faculty of Veterinary Medicine of the Universidad Nacional Mayor de San Marcos in Peru, we checked the efficacy of a vaccination program of three doses of HIPRAVIAR® CLON (using different administration routes), against a challenge with the NDV regional genotype involved in outbreaks. The challenge strain of NDV used was Chicken/Arequipa-Peru/VFAR81/2015 previously isolated in Peru and characterised as velogenic, genotype XII (Chumbe et al. 2017).
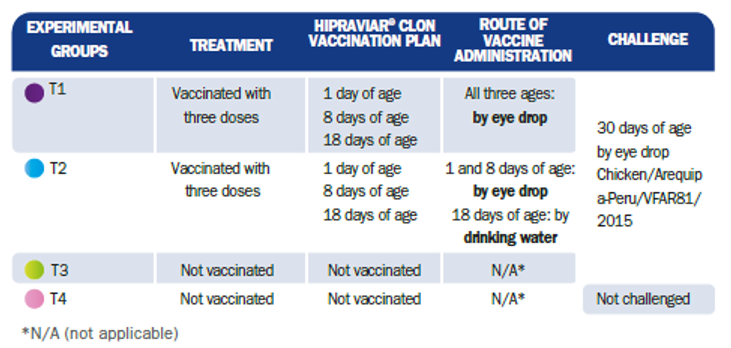
A total of 120 male 1-day-old broiler chicks from the Ross 308 line were selected, in good health and from the same batch of breeders free of Mycoplasma gallisepticum and Mycoplasma synoviae. The birds in the study were distributed between four experimental groups of 30 animals each (Table 1.).
Table 1. Study design and identification of experimental groups
Two groups (T1 and T2) were vaccinated with HIPRAVIAR® CLON at 1, 8 and 18 days of age, via drinking water or by eye drop (Table 1). The remaining groups (T3 and T4) were not vaccinated.
Groups T1, T2 and T3 were challenged at 30 days of age with 50 μl of an inoculum of Chicken/Arequipa-Peru/VFAR81/2015 with 106 EID50 administered by eye drop. Group T4 was not challenged (Table 1). During the test, clinical signs, mortality and nervous sequelae were evaluated.
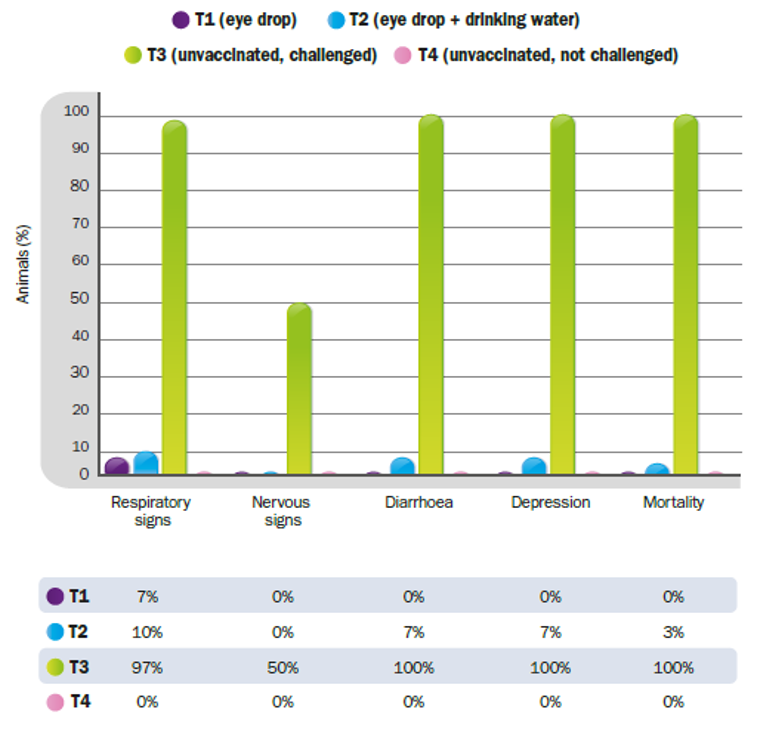
The two HIPRAVIAR® CLON vaccination programs evaluated in this study demonstrated sufficient efficacy to significantly prevent mortality and reduce morbidity; in particular, the variety and frequency of clinical signs induced by the Chicken/Arequipa-Peru/VFAR81/2015 genotype XII velogenic strain of NDV in broilers. In particular, a 97% reduction in mortality was observed for the mixed administration regimen (drinking water and eye drop) and up to 100% for the program based on eye drop administration.
- In group T1 (HIPRAVIAR® CLON, 1, 8 and 18 days by eye drop), none of the birds died after the challenge, while a small percentage (7%) developed clinical respiratory signs (Figure 1.).
- In group T2 (HIPRAVIAR®CLON, 1 and 8 days by eye drop, and 18 days by drinking water), a 3% mortality rate was observed after the challenge and respiratory clinical signs, diarrhoea (Image 1.) and depression were observed in a small number of birds (7-10%) (Figure 1.).
- In group T3 (Not vaccinated, challenged), a 100% mortality rate was observed (confirming the high pathogenicity of the viral strain used in the challenged groups), as well as clinical respiratory and nervous signs (paralysis, torticollis and tics), diarrhoea (Image 1.) and depression in most animals (50-100%). In the control group (T4), no mortality or clinical signs were observed (Figure 1.).
Image 1. General appearance of the feces after the challenge of the birds with the Chicken/Arequipa-Peru/VFAR81/2015 NDV strain.
Figure 1. Clinical signs and mortality after the challenge with Chicken/Arequipa-Peru/VFAR81/2015.
HIPRAVIAR® CLON/H120 performance against NDV genotype VII.
In order to reduce costs, the combined vaccination against NDV and IB has become common practice in the poultry industry. For the prevention of ND and IB, we also checked the efficacy of a commercial vaccine HIPRAVIAR® CLON / H120 against a challenge with genotype VII ND virus.
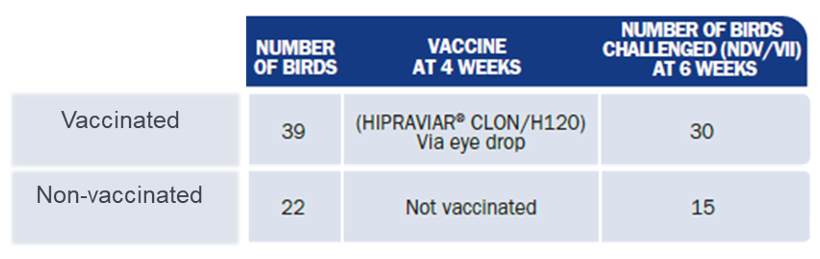
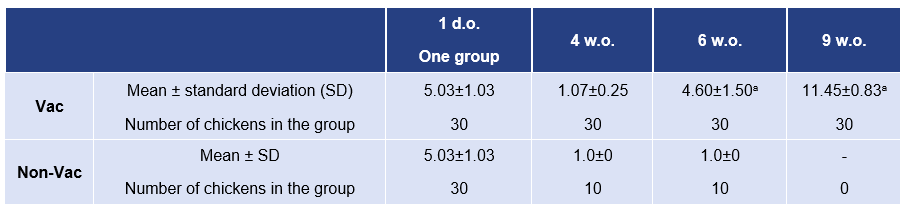
The study was conducted at Chulalongkorn University in Bangkok, Thailand. This study comparatively assessed the efficacy of a NDV/IB vaccine (HIPRAVIAR® CLON/H120) in commercial layers versus a non-vaccinated group. Efficacy was assessed by evaluating the serological response and protection against a challenge with NDV, genotype VII (orally, dose 105 EID50) two weeks post-vaccination (6 weeks of age). (Table 2.).
- Vaccinated group with 39 birds, which were vaccinated by eye drop at 4 weeks old with a combined freeze-dried vaccine (HIPRAVIAR® CLON/H120, dose >106.5 EID50).
- Non-vaccinated group with 22 birds, which were not vaccinated (negative control).
Table 2. Trial design
The following parameters were measured:
1) Post-challenge mortality
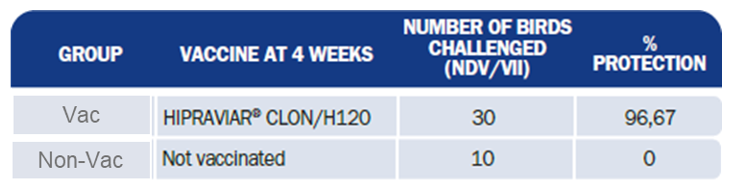
Mortality in the non-vaccinated (Non-Vac) group was 100% (0% protection) whilst in the vaccinated group (Vac) it was 3,33% (96,7% protection) 2 weeks after challenge, showing a significant protection provided by HIPRAVIAR® CLON when compared with the non-vaccinated group. (Table 3.).
Table 3. Percentage (%) mortality of laying birds vaccinated or not vaccinated and challenged with NDV genotype VII.
2. Serology - hemagglutination inhibition test (HI) for NDV
Titers for maternal and post-vaccination NDV antibodies were established by HI (Table 4.), and the birds vaccinated with HIPRAVIAR® CLON/H120 were efficiently protected when they were challenged with NDV genotype VII (Table 4.). This was confirmed by the high antibody titers against NDV obtained after vaccination and the protection against the challenge.
Table 4. HI antibody titers (log2) against NDV.
This experiment confirmed the efficacy of the use of the combined vaccine HIPRAVIAR® CLON/H120 for the control of Newcastle Disease in commercial layers against a controlled challenge with ND virus genotype VII, when using one dose and challenged 2 weeks after vaccination.
Discussion
Some authors have shown that vaccines based on strains related to LaSota can reduce mortality and clinical signs caused by different genotypes such as III, IV, V, VI, VII and IX (Liu et al. 2003, Jeon et al. 2008, Cornax et al. 2012, Miller et al. 2013, Bhuvaneswari et al. 2017). This evidence suggests that HIPRAVIAR® CLON and others based on LaSota could have a wide range of heterologous or even universal protection against NDV.
The type of vaccine and the route of administration may be vital for the correct immunization of birds. Other authors have demonstrated that both the antigenic dose and the route of administration can have a significant influence on the degree of protection or on the type of immune response induced against NDV (Al- Garib et al. 2003, Sasipreeyajan et al. 2006, Cornax et al. 2012, Dimitrov et al. 2016). A previous study showed that to obtain protection against genotype VII, a minimum titer of genotype II vaccine of 104 EID50 was needed (Cornax et al, 2012).
However, as can be seen in Figure 1, the two vaccination plans tested in this study (groups T1 and T2) showed slight differences in efficacy between them; in particular, it was observed that changing the route of administration from ocular to oral, in the last dose of the vaccination plan, decreased both the effectiveness of protection against mortality (0% only with ocular administration versus 3% with ocular and oral administration) and the efficacy in prevention of the appearance of clinical signs (7% in the group vaccinated only by eye drop versus 10% in the group vaccinated by eye drop and drinking water). These results could be explained by the fact that ocular administration has a greater antibody response and better protection against exposure to NDV compared to the oral route, as other authors have previously observed (Sasipreeyajan et al. 2006), but we should not rule out the effect of the vaccination methodology, since a vaccination program with 100% individual application is compared with a vaccination program with 2 individual doses and a dose given by mass application.
Conclusions
Cloned live vaccines against NDV, such as HIPRAVIAR® CLON or HIPRAVIAR® CLON/H120, continue to be a great option in the control of Newcastle disease in broilers. They are a safe and effective prophylactic tool, even in the face of genetic diversity of NDV. Nevertheless, this genetic variability will continue to be one of the concerns of poultry production, since genotyping and classification based on the NDV genotype is relatively recent, and more tests are needed to ascertain the most appropriate vaccination strategies against each Newcastle virus. The results of these tests provide yet further evidence that HIPRAVIAR® CLON and HIPRAVIAR® CLON/H120, when used correctly in a vaccination strategy designed according to the degree of challenge, are able to prevent mortality and reduce clinical signs induced by genotypes XII and VII NDV.
HIPRAVIAR® CLON and HIPRAVIAR® CLON/H120 are vaccines designed with a viral titer of 106.5 EID50; this characteristic could have been a relevant factor for the correct development of heterologous protection in this and other studies pertaining to the same vaccines.
References
Al-Garib, S. O., A. L. Gielkens, D. E. Gruys, L. Hartog, and G. Koch. 2003. Immunoglobulin class distribution of systemic and mucosal antibody responses to Newcastle disease in chickens. Avian Dis. 47: 32-40.
Bhuvaneswari, S., K. G. Tirumurugaan, P. Venkatesan, K. P. Manesh, and K. Kumanan. 2017. Evaluating the efficacy of LaSota vaccination induced protection in chickens upon challenge with a genotype IV strain of Newcastle disease virus. Virus disease. 28: 328-336.
Chumbe, A., R. Izquierdo-Lara, L. Tataje, R. Gonzalez, G. Cribillero, A. E. Gonzalez, M. Fernandez-Diaz, and E. Icochea. 2017. Pathotyping and Phylogenetic Characterization of Newcastle Disease Viruses Isolated in Perú: Defining Two Novel Subgenotypes Within Genotype XII. Avian Dis. 61: 16-24.
Cornax, I., P. J. Miller, and C. L. Afonso. 2012. Characterization of live LaSota vaccine strain-induced protection in chickens upon early challenge with a virulent Newcastle disease virus of heterologous genotype. Avian Dis. 56: 464-470.
Dimitrov, K. M., C. L. Afonso, Q. Yu, and P. J. Miller. 2016. Newcastle disease vaccines-A solved problem or a continuous challenge? Vet. Microbiol.
Jeon, W. J., E. K. Lee, Y. J. Lee, O. M. Jeong, Y. J. Kim, J. H. Kwon, and K. S. Choi. 2008. Protective efficacy of commercial inactivated Newcastle disease virus vaccines in chickens against a recent Korean epizootic strain. J. Vet. Sci. 9: 295-300.
Liu, X. F., H. Q. Wan, X. X. Ni, Y. T. Wu, and W. B. Liu. 2003. Pathotypical and genotypical characterization of strains of Newcastle disease virus isolated from outbreaks in chicken and goose flocks in some regions of China during 1985-2001. Arch. Virol. 148: 1387-1403.
Miller, P. J., C. L. Afonso, A. J. El, K. M. Dorsey, S. C. Courtney, Z. Guo, and D. R. Kapczynski. 2013. Effects of Newcastle disease virus vaccine antibodies on the shedding and transmission of challenge viruses. Dev. Comp Immunol. 41: 505-513.
Sasipreeyajan, J., Areeraksakul, P., Khanda, S. Protective Efficacy of Live LaSota Strain Newcastle Disease Virus Vaccine in Layer-Type Chickens. Thai J. Vet Med.46 (2): 195-200, 2006.