Evaluation of the combined effect of probiotics and coccidia vaccine in coccidia-challenged broilers
Coccidiosis is a high-risk infection in modern commercial poultry systems, as well as being capable of causing considerable economic losses to any medium- and large-scale operation. To minimise the effect of this parasitic disease, veterinary drugs such as coccidiostats have been employed. However, there are concerns about enteric pathogen resistance because of the prolonged use of these drugs. Therefore, new tools, such as anticoccidial vaccines and probiotics have drawn the attention of poultry producers.By Martina Dardi1, Marc Pagès1, Basharat Syed2, Luis Valenzuela2
(1HIPRA, Avda. La Selva 135, 17170, Amer (Girona), Spain. 2Biomin Holding GmbH, Erber Campus 1, 3131 Getzersdorf, Austria. 55th Symposium of the Spanish Branch of the WPSA (AECA), Madrid, Spain)
Download the poster publication here
Introduction
Coccidiosis is a significant ubiquitous disease in poultry with a huge economic impact on the poultry industry. The use of chemotherapeutics to control the disease has been successful in many parts of the world. However, there is increased pressure on the industry to reduce the dependence on these compounds due to their erratic effectiveness and the growing demand for poultry products free from any chemotherapeutics.
Vaccines and probiotics are considered two of the novel methods to control the disease without dependence on anticoccidial drugs, better known as coccidiostats. In addition, for these reasons, the use of anticoccidial vaccines has increased significantly. Furthermore, live coccidiosis vaccines have the advantage of providing good and long-lasting protection against coccidian invasion, together with a known capability of restoring sensitivity against coccidiostats.
There are live non-attenuated and attenuated vaccines on the market. Live non-attenuated vaccines consist of parasites that still maintain their natural virulence. Control of the development of adverse reactions (coccidiosis disease) is achieved through the use of low numbers of oocysts in vaccine preparations and in some cases even by the use of coccidiostats to control the excessive spread of vaccine strains. On the other hand, live attenuated vaccines are specifically designed to generate an immune response whilst limiting the threat of possible adverse events.
The most widely used attenuation system is the selection of strains by precocious development (1). Additionally, supplementation of probiotics in humans and animals has been shown to increase gut defence mechanisms against enteric pathogens. Probiotics are known as direct-fed microbials and are classified as live non-pathogenic microorganisms that are capable of maintaining a normal gut microbial population (2, 3). Probiotics have been used extensively in poultry production for their benefits in terms of performance, protection against enteric diseases and immunity of the birds (4).
The early introduction of non-pathogenic microorganisms is more effective for the establishment of the digestive tract, since gut microbiota begin to establish within hours after the chick hatches (5, 6). Through their multiple modes of action comprising pathogen antagonism, competitive exclusion and stimulation of the immune system, probiotics help to maintain a healthy balance of the gut microorganisms (6, 7). Moreover, multi-species probiotics may be able to attenuate any stress from early vaccine administration.
In this research, the added protective effect of the synbiotic (synergistic combination of probiotics plus one prebiotic), PoultryStar® (PoultryStar, BIOMIN GmbH, Austria) containing Enterococcus sp., Bifidobacterium sp., and Lactobacillus sp., plus fructooligosaccharide (FOS) derived from inulin was assessed in broilers vaccinated against coccidiosis with a live attenuated vaccine, HIPRACOX® (marketed by HIPRA and containing precocious sporulated oocysts of Eimeria acervulina 003, E. maxima 013, E. mitis 006, E. praecox 007 and E. tenella 004) immediately after hatching and challenged with a coccidia species mixture at day 15.
Materials and methods
Birds and treatments
456 day-old (DOC) male broilers of the ROSS 308 breed were housed for a 35-days grow-out in floor pens covered with wood shavings in a thickness of about 5 cm. One commercial pan feeder with a feed reservoir and four drinking nipples was suspended on the inside of the pen. Ventilation and heating were regulated automatically. The pens were made out of materials not detrimental to the health of the birds. Their design and construction was in accordance with Directive 2010/63/EU so that no injury could be caused to the animals. They were made out of materials that withstand cleaning and decontamination techniques. Pelleted feed and water were provided ad libitum. Commercial feed contained neither antimicrobials nor anticoccidial additives. The animals were divided into 3 treatment groups of 152 animals with 8 replicates per group (shown in Table 1).
Table 1. Trial Groups
The HCPS group received a HIPRACOX® vaccination on the farm on arrival day via drinking water in bell drinkers and PoultryStar® sol (20 mg/bird/day) via drinking water with bell drinkers for the first 3 days. Moreover, PoultryStar® me was applied via feed 1 kg/ton during the starter phase (1-14) and 0.5 kg/ton during the grower phase (15-35).
Eimeria challenge
The following inoculum was used on day 15 to inoculate the birds of the IUC and HCPS groups (at 1ml/bird) (Table 2). The Eimeria strains used for the challenge were pathogenic strains isolated in Germany.
Table 2. Inoculum for Eimeria challenge
On days 21 and 22 (6 and 7 days post-challenge) 2 birds per pen were randomly selected, individually weighed and humanely euthanized. Lesion scores were assessed for E. acervulina, E. maxima and E. tenella by the method of Johnson & Reid (8). The lesion scores were then recorded as the average across the two birds for each segment. The total lesion score was calculated as the sum of lesion scores in the three intestinal segments (duodenum, mid-intestine, caecum). Faeces samples were collected from each group for the oocyst count per gram on days 6 and 7 post-vaccination and 7 and 14 days post-challenge. Fresh faecal droppings were collected and pooled for each group. Samples from each group were placed in separate airtight plastic bags, homogenised thoroughly by a domestic mixer, and kept refrigerated until assessed for oocyst counts by the McMaster method. Oocyst numbers were determined by dilution and microscopic counting and expressed as oocysts per gram (OPG) of excreta.
Performance
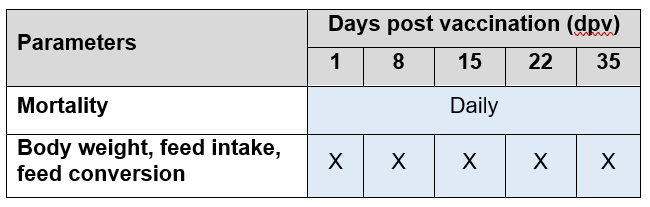
Productive parameters were recorded as shown in Table 3.
Table 3. Summary of recorded productive parameters
Statistical analysis
The statistical analysis methodology was in accordance with those outlined in the WAAVP guidelines for evaluating the efficacy of anticoccidial drugs in chickens and turkeys (9). Data were analysed with R. Data on body weight (BW), daily weight gain (DWG), daily feed intake (DFI), feed conversion ratio (FCR), total intestinal lesion score (ILS) and OPG using a linear regression model with treatment group as fixed effect (lm procedure of the core package). A natural logarithmic transformation [Ln(x+1)] was performed on the OPG data to obtain a normalised distribution. If high mortality was observed, mortality was analysed using cox proportional hazard models (coxph procedure of the package survival). P-values of the ordered regression models were calculated by comparing the t-value against the standard normal distribution using a z test. Residual plots were checked to evaluate the model fit. Statistical significance was assessed at P ≤ 0.05.
Results
Lesion scores
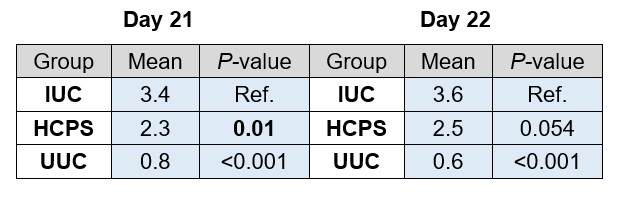
Table 4 shows the total mean coccidiosis score per study day and group. On day 21, a significant difference in lesion score was detected between the IUC and the HCPS group, in fact the HCPS birds showed significantly lower lesion scores compared to the IUC (P < 0.05). Whereas, on day 22, the lesion score of the HCPS group was numerically lower compared to the IUC group, but with no statistical significance.
Table 4. Total Mean Coccidiosis Score: Day 21, Day 22. Differences were analysed with linear regression models with treatment as categorical fixed effect (lm procedure of the core package). Statistics for the HCPS and UUC groups were calculated Vs the IUC group.
OPG
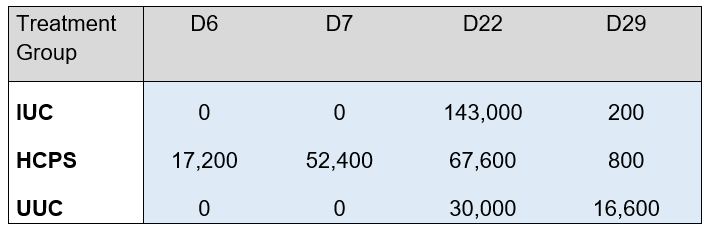
Table 5 shows the OPG in oocysts per gram per group and study day. The IUC group shows zero OPG counts on days 6 and 7 with a pronounced increase on day 22 (143,000) –as this was the untreated challenge group- as well as a decrease on day 29, which reveals that after the challenge the birds acquired immunity and decreased oocyst output. Similarly, the UUC group showed zero oocyst output on days 6 and 7 together with some levels of OPGs on days 22 and 29, revealing that -after the challenge- likewise a cross-contamination occurred in the untreated unchallenged birds. Finally, the HCPS group shows, as expected, vaccine replication OPG levels on days 6 and 7. Then, on day 22, oocyst output is less than half (67,600) of the level shown by the IUC group, revealing that birds were already immunised and well protected at the time of the challenge, and on day 29 the OPG level was as low as that of a flock that is no longer shedding oocysts thanks to immunisation.
Table 5. Total OPG
Performance
Mortality
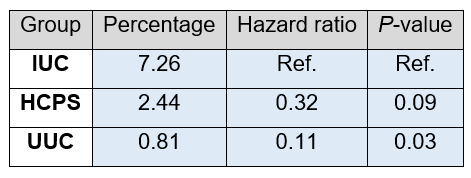
Even if there is no statistically significant difference, mortality in the HCPS group was numerically far lower compared to the IUC group.
Table 6. Mortality. Differences in total mortality were analysed using a cox proportional hazard model. The table below shows the percentage mortality, hazard ratios and the P-value. Statistics for the HCPS and UUC groups were calculated Vs the IUC group.
Mean Body Weight (BW), Daily Weight Gain (DWG), Daily Feed Intake (DFI), Feed Conversion Ratio (FCR)
Results for productive parameters are shown in Table 7.
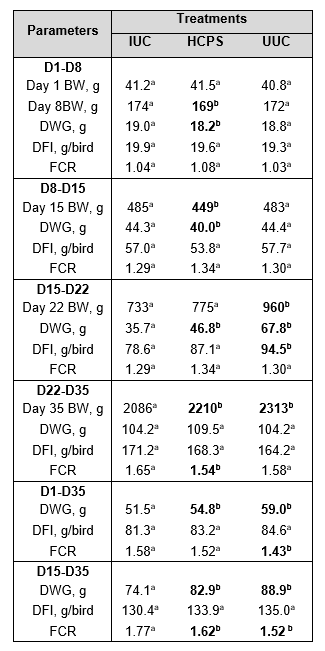
Table 7. Productive parameters. Differences were analysed with linear regression models with treatment as categorical fixed effect (lm procedure of the core package).
a-b Means within rows that do not have the same superscript as the IUC group differ significantly (P ≤ 0.05) from this reference group.
On day 1, no significant differences were observed between the different treatment groups and the IUC group. On day 8, birds from the HCPS group weighed significantly less compared to birds from the IUC group, however, the differences were only numerical. On day 15 (before inoculation), significant lower body weight was seen in the HCPS group, probably caused by the replication of the vaccine. On day 22 (after inoculation), birds from the UUC group weighed significantly more compared to birds from the IUC group. At this point there was no significant difference between the HCPS and the IUC group, indicating a compensatory growth. On day 35, birds from the HCPC group and the UUC group weighed significantly more compared to birds from the IUC group.
From days 1-8 and from days 8-15, birds from the HCPS group gained significantly less weight compared to birds from the IUC group, probably caused due to stress generated by replication of the vaccine. During the phase covering days 15-22 (acute phase after inoculation), birds from the HCPS group and the UUC group gained significantly more weight compared to birds from the IUC group. Between days 22-35 (recovery period), no significant differences were observed. Overall, from days 1-35 and days 15-35, birds from the HCPS group and the UUC group gained significantly more weight compared to birds from the IUC group.
In any of the periods, days 1-8, 8-15, 22-35, 15-35 and overall 1-35, no significant differences were observed amongst the three groups with regard to daily feed intake. Only between days 15-22 did birds from the UUC group have a higher daily feed intake compared to birds from the IUC group.
The periods covering days 1-8, 8-15 and 15-22 showed no significant difference amongst the three groups regarding FCR. In the period covering days 22-35, both groups had a lower FCR compared to the IUC group, although this was only significant for the HCPS group. In the periods covering days 1-35 and 15-35, birds from the HCPS and UUC group had a lower FCR compared to birds from the IUC group.
Discussion
In this research, the added protective effect of a synbiotic was noticeable in broilers vaccinated against coccidiosis immediately after hatching and challenged with a mixed coccidia challenge infection at day 15. The challenge with Eimeria species seemed to be successful, as birds from all inoculated groups had some degree of intestinal lesion scores for Eimeria spp. on days 21 and 22, except birds from the UUC group. On days 21 and 22, the lesion scores in birds from the HCPS group were lower compared to the IUC group. In addition, the birds treated with HCPS could suppress the oocyst shedding more than the IUC birds. When comparing performance parameters, data for birds from the UUC and IUC groups showed that the coccidiosis infection had an impact on the overall performance of the birds. When considering the entire study period, birds from the UUC group performed significantly better compared to birds from the IUC group regarding body weight, weight gain and FCR. Birds treated with HIPRACOX® + PoultryStar® performed better compared to birds from the IUC groups regarding weight gain and FCR. In the pre-challenge period (days 1-15), the daily weight gain was lower in the birds from the HCPS group potentially due to the replication of the vaccinal strains, but due to the compensatory growth afterwards this had no impact on the final weight of the birds at the end of the study (day 35).
Conclusions
In conclusion, results from the present study showed that the combination of HIPRACOX® and PoultryStar® had a positive impact on the zootechnical performance of the birds and on the coccidiosis lesion scoring after experimental induction of coccidiosis. This suggests a beneficial effect of the combination of HIPRACOX® and PoultryStar® on digestion and overall gut health.
References
1. Jeffers T.K. (1975). Attenuation of Eimeria tenella through selection for precociousness. J. Parasitol. 61 (6):1083-90.
2. Patterson JA, Burkholder KM (2003). Application of prebiotics and probiotics in poultry production. Poult Sci 82:627–631.
3. Ohimain EI, Ofongo RTS (2012). The effect of probiotic and prebiotic feed supplementation on chicken health and gut microflora: a review. Int J Anim Vet Adv 4:135–143.
4. Lutful-Kabir S. M. (2009). The role of probiotics in the poultry industry. Int. J. Mol. Sci. 10:3531–3546.
5. Timmerman H.M., Veldman A., van den Elsen E., Rombouts F.M., Beynen A.C. (2006). Mortality and growth performance of broilers given drinking water supplemented with chicken-specific probiotics. Poult. Sci. 85:1383–1388.
6. Torok V.A., Ophel-Keller K., Hughes R.J., Forder R., Ali M., Macalpine R. (2007). Environment and age: impact on poultry gut microflora. Aust. Poult. Sci. Symp .19:149–152.
7. Cox CM, Dalloul RA (2015). Immunomodulatory role of probiotics in poultry and potential in ovo application. Benef Microbes 6:45–52.
8. Johnson J., Reid W.M. (1970). Anticoccidial drugs: lesion scoring techniques in battery and floor-pen experiments with chickens. Exp. Parasitol. 28: 30-36.
9. Holdsworth P.A., Conway D.P., McKenzie M.E., Chapman H.D., Mathis G.F., Skinner J.T., Mund H.-C., Williams R.B. (2004). World Association for the Advancement of Veterinary Parasitology (WAAVP) guidelines for evaluating the efficacy of anticoccidial drugs in chickens and turkeys. Vet. Parasitol. 121 (3-4):189-212.