



Heat stress in poultry
What oxidative stress has to do with it, why it affects gut health, and how phytomolecules support mitigation strategiesStress in animals can be defined as any factor causing disruptions to their homeostasis, their stable internal balance. Stress engenders a biological response to regain equilibrium (1). We can distinguish four major types of stress in the poultry industry: technological or management-related stress; environmental stress; nutritional stress, including due to heavy metals, mycotoxins, and low-quality ingredients; and internal stress, which is related to health status and health challenges. (2). All types of stress lead to molecular and cellular changes that decrease health and productivity.
Climate change, thermoregulation, and stress
High environmental temperatures are among the most important environmental stressors for poultry production, causing significant economic losses in the industry (3). Climate change has increased the prevalence and intensity of heat stress conditions in most poultry production areas all over the world (4, 5)
The optimum temperature for poultry animals well-being and performance – the so-called thermoneutral zone – is between 18 and 22°C. When birds are kept within this temperature range, they do not have to spend energy on maintaining a constant body temperature (6).
Heat stress is the result of unsuccessful thermoregulation in the animals, as they absorb or produce a higher quantity of heat than they can lose. It means that there is a negative balance between the net amount of energy flowing from the animal to the environment and the energy it produces (7).
Heat stress – contributing factors
This energy imbalance is influenced by environmental factors such as sunlight, thermal irradiation, air temperature, humidity, and stocking density, but also by animal-related factors such as body weight, feather coverage and distribution, dehydration status, metabolic rate, and thermoregulatory mechanisms (7, 8). When the environmental temperature is above the thermoneutral zone, the animals activate thermoregulation mechanisms to loose heat through behavioral, biochemical, and physiological changes and responses (9-12).
Heat stress can be classified into two main categories, acute and chronic. Acute heat stress refers to a short and fast increase in environmental temperature (a few hours), whereas under chronic heat stress the high temperatures persist for more extended periods (several days). Some studies suggest that, in some circumstances, poultry animals show a degree of resilience to acute heat stress (7, 9, 10). However, in the long-run, their compensatory mechanisms are not sufficient to maintain tissue integrity and thus health and performance (11)
The animal’s response to heat stress
The exposure of poultry to heat stress changes the gene expression of cytokines, upregulates heat shock proteins (HSP), and reduces the concentration of thyroid hormones (10, 12). When heat stress persists, these cascades of cellular reactions result in tissue damage and malfunction.
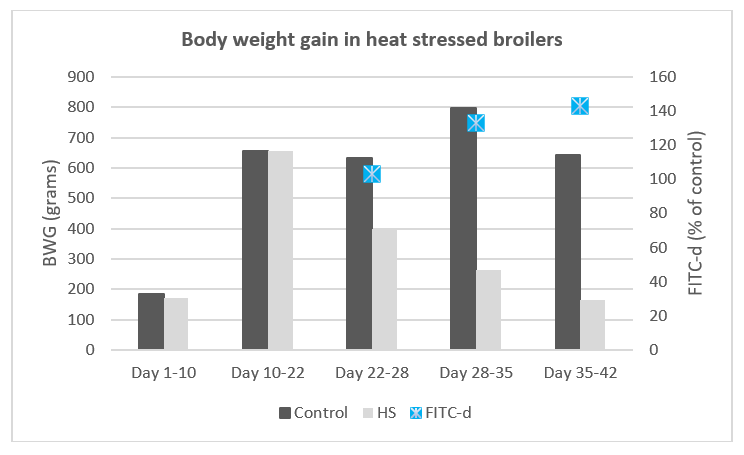
The animals exposed to heat stress suffer adverse effects in terms of performance, which are widely known and include high mortality, lower growth and production (Figure 1), and a decline in meat and egg quality (13, 14).
Oxidative stress – a consequence of heat stress
Oxidative stress, simply put, occurs when the amount of reactive oxygen species (ROS – such as superoxide anions, hydrogen peroxide, and hydroxyl radicals) exceeds the antioxidant capacity of the cells (6, 14, 15). Oxidative stress is regarded as one of the most critical stressors in poultry production as it is a response to diverse challenges affecting the animals (2, 17)
At a cellular level, the metabolism of the animal – its energy production – generates ROS and reactive nitrogen species (RNS), such as hydroxyl radicals, superoxide anions, hydrogen peroxide, and nitric oxide. These usually are further processed by antioxidant enzymes produced by the cell (2, 15), including superoxide dismutase (SOD), catalase (CAT), and glutathione peroxidase (GSH-Px). Nutrients such as selenium and vitamins E, C, and A also participate in antioxidant processes (2, 5). When the generation of ROS exceeds the capacity of the antioxidant system, oxidative stress ensues (2, 16).
Heat stress leads to higher cellular energy demand, promoting the generation of ROS in the mitochondria (13), which exceed the antioxidant capacity of the organism. As a consequence, oxidative stress occurs in several tissues, leading to cell apoptosis or necrosis (11). Among these tissues, the gastrointestinal tract can be highly affected.
Oxidative stress damages cell proteins, lipids, and DNA, and reduces energy generation efficacy (6). Moreover, oxidized molecules can take electrons from other molecules, resulting in a chain reaction. If not controlled, this reaction can cause extensive tissue damage (16).
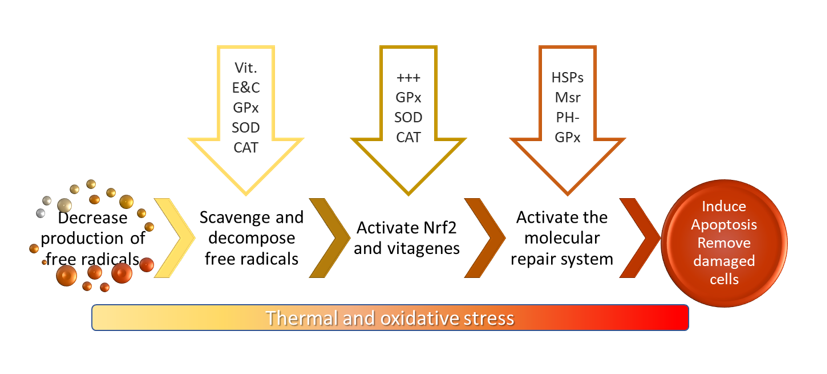
In response to oxidative stress, all antioxidants in the organism work together to re-establish homeostasis. Several steps in the oxidative stress response have been identified. Whether they take place depends on the intensity of the stressor, with ROS and RNS acting as signalling molecules. These steps include the internal synthesis of antioxidants, the activation of transcription factors or vitagenes, and the production of protective molecules (Figure 2).
Oxidative stress’ effects on the gut
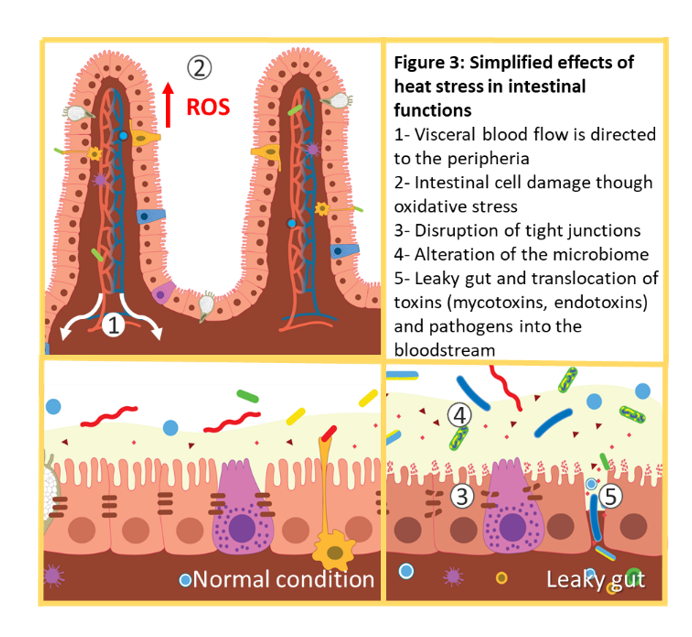
In the gastrointestinal tract, oxidative stress and the consequent tissue damage lead to increased intestinal permeability. This facilitates the translocation of toxins and pathogens from the intestinal tract into the bloodstream (Figure 3).
Under oxidative stress conditions in the gut, there is a demand for antioxidants to counteract the excess of ROS; hence, dietary antioxidants can help reduce ROS and improve animal performance (15). Research shows that certain phytomolecules have antioxidant properties and improve performance under conditions of oxidative stress (14, 18-20).
Thermoregulation: changes in blood flow
The gastrointestinal tract is profoundly affected by heat stress: to help with heat dissipation, the thermoregulatory mechanism of the animal shifts visceral blood flow towards peripheral circulation. Organ ischemia and hypoxia follow, limiting gut motility, nutrient utilization, and feed intake (5, 14). Enterocytes are particularly sensitive to hypoxia and nutrient restriction, which leads to oxidative stress (2, 12).
Changes in intestinal barrier’s tight junctions
Several studies indicate that both acute and chronic heat stress increase gut permeability, partly by increasing oxidative stress and by disrupting the expression of tight junction proteins (5, 21). Heat and oxidative stress in the gut result in intestinal cell injury and apoptosis. When the tight junction barrier is compromised, luminal substances leak into the bloodstream, which constitutes the condition described as “leaky gut”(4, 21).
Changes in intestinal morphology
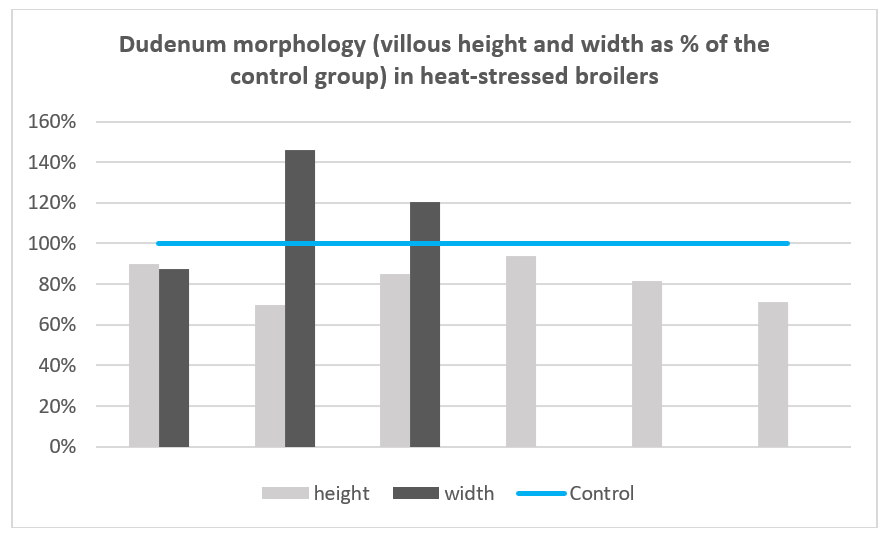
Heat stress affects intestinal weight, length, barrier function, and microbiota, resulting in animals that have lower total and relative weight of the small intestine, with shorter jejunum and duodenum, shorter villi (Figure 4), and reduced absorption areas, in comparison to non-stressed animals (11, 12, 23-26).
Changes in intestinal microbiome
Due to reduced feed intake and impaired intestinal function, the presence and activity of the commensal microbiota can also be modified. Heat stress can lead to reduced populations of beneficial microbes. At the same time, it can boost the growth of potential pathogens and lead to dysbiosis, increased gut permeability, as well as immune and metabolic dysfunction (27). Burkholder et al. (2008) and Rostagno (2020) point out that pathogens such as Clostridia, Salmonella, and coliform bacteria increase in poultry exposed to heat stress, while the populations of beneficial bacteria such as Lactobacilli and Bifidobacteria decrease.
Necrotic enteritis
Heat stress causes damage in the gut microbiota, intestinal integrity, and villus morphology, as well as immunosuppression. Consequently, feed digestion and absorption decline (11, 12, 28, 29). These factors increase the risk of necrotic enteritis outbreaks (5, 28, 30, 31), one of the most problematic bacterial diseases in modern poultry production.
In a study by Tsiouris et al. (2018), cyclical acute heat stress was found to increase the incidence and severity of necrotic enteritis in broilers challenged with C. perfringens, and to produce the disease in animals that were not exposed to the bacteria. Other signs, such as growth retardation and a reduced pH of the intestinal digesta, were also observed in the heat-stressed birds.
By lowering feed digestibility, increasing gut permeability, and compromising immunity, heat stress leaves animals more susceptible to gut-health related issues such as dysbacteriosis and necrotic enteritis – and thus increases the need to use antibiotics.
Mitigation strategies
Most intervention strategies deal with heat stress through a wide range of measures, including environmental management, housing design, ventilation, sprinkling, and shading, amongst others (8). Understanding and controlling environmental conditions is always a part of heat stress management: it is crucial for ensuring animal welfare and achieving successful poultry production.
Feed management and nutrition interventions are also recommended, together with environmental management, to reduce the effects of heat stress. They include feeding pelletized diets with increased energy, higher fat inclusions, reduction of total protein, supplemental amino acids, higher levels of vitamins and minerals, and adjusting the dietary electrolyte balance (1, 12, 18). Nutrition is crucial, and the use of the right diets aids in attenuating heat stress in birds.
Phytomolecules: powerful antioxidants
It is practically impossible to avoid stress in commercial poultry production; hence it is common for animals to experience oxidative stress at times. Phytomolecules are natural antioxidants with anti-inflammatory and digestive properties (8, 14), which have been shown to improve poultry performance, including during challenging periods. The antioxidant capacity of phytomolecules manifests itself in free radical scavenging, increased production of natural antioxidants, and the activation of transcription factors (2, 32, 33).
As compounds that have low bioavailability, they can remain at high concentrations within the intestine, when provided at the appropriate dosage and through encapsulation technology. Research has found that phytomolecules can effectively reduce intestinal ROS and thus alleviate heat stress in poultry (15, 18-20), specifically mitigating oxidative stress in the intestine.
One heat stress study, for example, found that carvacrol elevates serum GSH-PX activity, compared to non-supplemented broilers (19). Other studies demonstrate that cinnamaldehyde also increases the activities of natural antioxidants in heat-stressed broilers (32, 35). A study by Prieto and Campo (2016) showed that dietary supplementation of capsaicin effectively alleviated heat stress, as indicated by a lower H/L ratio in supplemented animals.
Silibinin, a flavonolignan present in silymarin (milk thistle extract), is another powerful antioxidant. In the gastrointestinal tract, it can come into direct contact with cells, activating transcription factors such as Nrf2, and thus helping to upregulate the antioxidant protection (34). Other phytomolecules, such as menthol and cineol, also aid animals under heat stress by simulating the sensory cold receptors of the oral mucosa. This gives them a cooling sensation and reduces heat stress behavior (18).
Summary
- Heat stress is a common reality in poultry production, its effects are quite complex and harmful and depend on the intensity and duration of the exposure to high temperatures.
- The gut is affected by heat stress through several pathways, including organ ischemia and hypoxia, as well as oxidative stress.
- In heat stress challenges, the intestinal barrier is compromised because of lower tight junction protein expression, enterocyte damage, and microbiome unbalance, leading to gut health issues such as dysbiosis and necrotic enteritis.
- At the gut level, phytomolecules such as carvacrol, cinnamaldehyde, capsaicin, silymarin, cineol, and menthol, among others, have been found to alleviate heat stress through their antioxidant capacities, leading to improved animal health and performance.
| References | ||||
|---|---|---|---|---|
| 1. | ||||
| (Das, S. et al., 2011. Nutrition in relation to diseases and heat stress in poultry. Veterinary World, 4(9), pp. 429-432.) | ||||
| 2. | ||||
| (Surai, P. F., Kochish, I. I., Fisinin, V. I. & Kidd, M. T., 2019. Antioxidant defence systems and oxidative stress in poultry biology: An update. Antioxidants, 8(7).) | ||||
| 3. | ||||
| (St-Pierre, N., Cobanov, B. & Schnitkey, G., 2003. Economic Losses from Heat Stress by US Livestock Industries. Journal of Daairy Science, Volume 86) | ||||
| 4. | ||||
| (Tellez Jr., G., Tellez-Isaias, G. & Dridi, S., 2017. Heat stres and gut health in broilers: role of tight junction proteins. Advances in Food Technology and Nutritional Sciences, 3(1).) | ||||
| 5. | ||||
| (Lian, P. et al., 2020. Beyond heat stress: intestinal integrity disruption and mechanism-based intervention strategies. Nutrients, Volume 12.) | ||||
| 6. | ||||
| (Akbarian, A. et al., 2016. Association between heat stress and oxidative stress in poultry; mitochondrial dysfunction and dietary interventions with phytochemicals. Journal of Animal Science and Biotechnology, 7(37).) | ||||
| 7. | ||||
| (Lara, L. & Rostagno, M., 2013. Impact of heat stress on poultry production. Animals, Volume 3, pp. 356-369.) | ||||
| 8. | ||||
| (Saeed, M. et al., 2019. Heat stress management in poultry farms: a comprehensive overview. Journal of Thermal Biology, Volume 84, pp. 414-425.) | ||||
| 9. | ||||
| (Farag, M. & Alagawany, M., 2018. Phyisiological alterations of poultry to the high enviromental temperature. Journal of Thermal Biology, Volume 76, pp. 101-106.) | ||||
| 10. | ||||
| (Quinteiro-Filho, W. et al., 2010. Heat stress impairs performance parameters, induces intestinal injury, and decreases macrohage activity in broiler chickens. Poultry Science, Volume 89, p. 1905–1914.) | ||||
| 11. | ||||
| (Santos, R. et al., 2015. Quantitative histo-morphometric analysis of heat-stress-related damage in the small intestines of broiler chickens. Avian Pathology, 44(1), pp. 19-22.) | ||||
| 12. | ||||
| (Awad, E. et al., 2018. Growth performance, duodenal morphology and the caecal microbial population in female broiler chickens fed glycine-fortified low protein diets under heat stress conditions. British Poultry Science, 59(3), pp. 340-348.) | ||||
| 13. | ||||
| (Mujahid, A., Yoshiki, Y., Akiba, Y. & Toyomizu, M., 2005. Superoxide radical production in chicken skeletal muscle induced by heat stress. Volume 84, pp. 307-314.) | ||||
| 14. | ||||
| (Hu, R. et al., 2019. Polyphenols as potential attenuators of heat stress in poultry production. Antioxidants, 8(67).) | ||||
| 15. | ||||
| (Salami, S. et al., 2015. Efficacy of dietary antioxidants on broiler oxidative stress, performance and meat quality: science and market. Avian Biology Research, 8(2), pp. 65-78.) | ||||
| 16. | ||||
| (Lauridsen, C., 2019. From oxidative stress to inflammation: redox balance and immune system. Poultry Science, Volume 98, pp. 4240-4246.) | ||||
| 17. | ||||
| (Surai, P. F. & Fisinin, V. I., 2016. Vitagenes in poultry production: Part 1. Technological and enviromental stresses. World's Poultry Science Journal, Volume 72.) | ||||
| 18. | ||||
| (Arab Ameri, S., Samadi, F., Dastar, B. & Zarehdaran, S., 2016. Efficiency of peppermint (Mentha piperita) powder on performance, body temperature and carcass characteristics of broiler chickens in heat stress condition. Iranian Journal of Applied Animal Science, 6(4), pp. 943-950.) | ||||
| 19. | ||||
| (Saadat Shad, H., Mazhari, M., Esmaeilipour, O. & Khosravinia, H., 2016. Effects of thymol and carvacrol on productive performance, antioxidant enzyme activity and certain blood metabolites in heat stressed broilers. Iranian Journal of Applied Animal Science, 6(1), pp. 195-202.) | ||||
| 20. | ||||
| (Mishra, B. & Jha, R., 2019. Oxidative stress in the poultry gut: potential challenge and interventions. Frontiers in Veterinary Science, 6(60).) | ||||
| 21. | ||||
| (Ruff, J. et al., 2020. Research Note: Evaluation of a heat stress model to induce gastrointestinal leakage in broiler chickens. Poultry Science, Volume 99, pp. 1687-1692.) | ||||
| 22. | ||||
| (Rostagno, M., 2020. Effects of heat stress on the gut health of poultry. Journal of Animal Science, 98(4).) | ||||
| 23. | ||||
| (Abdelqader, A. & Al-Fataftah, A., 2016. Effect of dietary butyric acid on performance, intestinal morphology, microflora composition and intestinal recovery of heat-stressed broilers. Livestock Science, Volume 183.) | ||||
| 24. | ||||
| (Jahejo, A. et al., 2016. Effect of heat stress and ascorbic acid on gut morphology of broiler chicken. Sindh University Research Journal, 48(4), pp. 829-832.) | ||||
| 25. | ||||
| (Wu, Q. et al., 2018. Glutamine alleviates heat stress-induced impairment of intestinal morphology, intestinal inflammatory response, and barrier integrity in broilers. Poultry Science, Volume 97, pp. 2675-2683.) | ||||
| 26. | ||||
| (Santos, R. et al., 2019. Effects of a feed additive blend on broilers challenged with heat stress. Avian Pathology, 48(6), pp. 582-601.) | ||||
| 27. | ||||
| (Shi, D. et al., 2019. Impact of gut microbiota structure in heat-stressed broilers. Poultry Science, Volume 98, pp. 2405-2413.) | ||||
| 28. | ||||
| (Burkholder, K. et al., 2008. Influence of stressors on normal intestinal microbiota, intestinal morphology, and susceptibility to Salmonella Enteritidis colonization in broilers. Poultry Science, Volume 87, pp. 1734-1741.) | ||||
| 29. | ||||
| (Quinteiro-Filho, W. et al., 2012. Acute heat stress impairs performance parameters and induces mild intestinal enteritis in broiler chickens: role of acute HPA axis activation. Journal of Animal Science.) | ||||
| 30. | ||||
| (Antonissen, G. et al., 2014. The Impact of Fusarium Mycotoxins on Human and Animal Host Susceptibility to Infectious Diseases. Toxins, 6(2).) | ||||
| 31. | ||||
| (Tsiouris, V. et al., 2018. Heat stress as predisposing factor for necrotic enteritis in broiler chicks. Avian Pathology, 47(6), pp. 616-624.) | ||||
| 32. | ||||
| (Abd El-Hack, M. et al., 2019. Herbs as thermoregulatory agents in poultry: An overview. Science of the Total Environment.) | ||||
| 33. | ||||
| (Surai, P. F., 2020. Antioxidants in poultry nutrition and reproduction: An update. Antioxidants, 9(2).) | ||||
| 34. | ||||
| (Surai, P. F., 2015. Silymarin as a natural antioxidant: An overview of the current evidence and perspectives. Antioxidants, 4(1).) | ||||
| 35. | ||||
| (El-Maaty, A., Hayam, M., Rabie, M. & El-Khateeb, A., 2014. Response of heat-stressed broiler chicks to dietary supplementation with some commercial herbs. Asian Journal of Animal and Veterinary Advances, 9(12), pp. 743-755.) | ||||
| 36. | ||||
| (Prieto, M. & Campo, J., 2010. Effect of heat and several additives related to stress levels on fluctuating asymmetry, heterophil:lymphocyte ratio, and tonic immobility duration in White Leghorn chicks. Poultry Science, Volume 89, p. 2071–2077.) | ||||









