Histomoniasis: what the experts say
An overview of transmission, diagnosis, control and preventionIntroduction
Histomoniasis or blackhead is a complex disease process. Although primarily affecting turkeys with lesions in the ceca and liver, blackhead can also have a significant impact on chickens (where lesions are often confined to the ceca only).
Blackhead is caused by the protozoan flagellate, Histomonas (H.) meleagridis, which has a broad host range, infecting gallinaceous birds including, pheasants, partridges, and bobwhite quails, in addition to chicken and turkeys.
With the ban on many of the drugs used to fight the disease, and changes in animal husbandry like reusing litter and/or increased stocking density, blackhead has re-emerged in many areas including North America and Europe. The focus for control of blackhead is now on prevention and the use of new diagnostic methods to better understand how to manage and eradicate the disease.
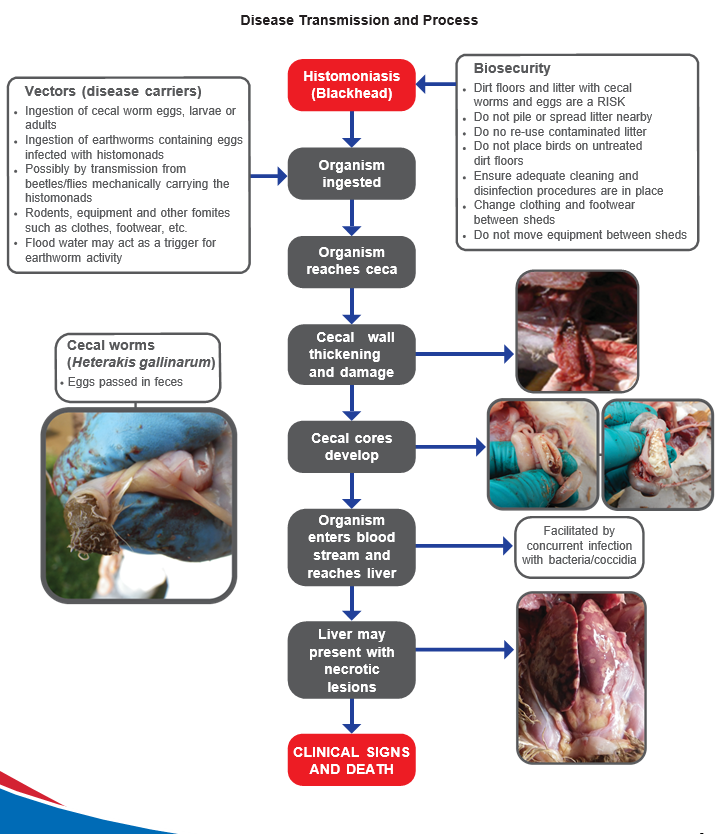
Vectors (carriers of disease) and transmission
- Ingestion of the adult common cecal worm (Heterakis gallinarum) or their embryonated ova (eggs) infected with H. meleagridis. Heterakis gallinarum is the only worm known to serve as an intermediate host for blackhead. After a series of divisions, a uniquely adapted, very small form of H. meleagridis actively invades the reproductive tract of the cecal worm and is subsequently shed within the infected worm egg. Cecal worm eggs are extremely resistant to environmental conditions and may remain infective for 2-3 years; there is some anecdotal/circumstantial evidence of a link between ground works (e.g. disturbing the litter to de-cake or during clean out or any other general disturbance of the ground) and histomonas breaks (the ground works may recirculate buried caecal worm eggs).
- Transport of the infected cecal worm egg by one of several other potential disease carriers or transport hosts. These include earthworms (which may ingest the eggs) or mechanical disease carriers such as flies or rodents which may simply transport the sticky eggs on their bodies. PCR (Polymerase chain reaction; a technique that allows analysis of DNA) has shown that black/darkling beetles (Alphitobius diaperinus) may contain H. meleagridis DNA, providing evidence of their role as a potential vector. People and equipment can also act as disease carriers and flood water may act as a trigger for increased earthworm activity.
- Direct transmission of H. meleagridis through cloacal drinking. Proven in turkeys only, this may be the reason why spread of disease appears to be more rapid in turkeys compared to chickens. Experimental direct oral inoculation of H. meleagridis (direct administration of the protozoa itself) has low success due to the parasites’ susceptibility to the acidic crop and gizzard. However, when H. meleagridis is administered in turkeys intracloacally or via “cloacal drinking” blackhead disease manifests.
Outside the host (or intermediate host: Heterakis gallinarum), H. meleagridis has a low persistence and survival time, although it has been shown to survive for up to 9 hours in water or faecal material. There is also some evidence that a cyst like stage can develop allowing the protozoa to survive for extended periods in the environment, although this is as yet unproven.
Clinical signs and diagnosis
Turkeys suffering from blackhead disease show ruffled feathers, drooped wings, apathy and sulphur coloured (yellow) droppings. Clinical signs in chickens may be less clear than in turkeys, or even go unnoticed, but can result in high mortality. Cecal discharge (droppings) may contain blood. Uniformity may be affected by a blackhead challenge in rear and, in lay, a drop in egg production may occur.
Clinical signs normally develop 7-14 days after infection although in experimental infections pathology can be seen between 5 and 19 days post infection. Field observations suggest that co-infection with coccidia, mostly E. tenella, may increase clinical signs. Initially the ceca will become swollen with a thickened wall; in more advanced cases, cecal cores develop (accumulations of clotted blood and tissue). Liver lesions are highly variable, but typically manifest as circular depressed target-like areas up to 1 cm/0.4 inch in diameter. However, in chickens, liver lesions do not always develop despite the development of cecal cores.
If blackhead is suspected, birds should be submitted to a diagnostic lab for post mortem examination.
- Macroscopically (via necropsy/autopsy). It is important to differentiate between infections with agents such as salmonellosis and coccidiosis as the lesions created by these infections can be easily mistaken for Histomoniasis lesions (all three entities can produces cecal cores). However, cecal lesions together with liver lesions are representative of a blackhead infection.
- Microscopically. The protozoa can easily be found in affected ceca and livers. This should be confirmed with histopathology (taking tissues samples to confirm the presence of histomads), at least for the first case in an outbreak.
Studies within Europe have found two different genotypes of H. meleagridis - Genotype 1 and Genotype 2. The differences between these two genotypes and the effect this has on the severity or harmfulness of the disease (virulence) between and within the genotypes needs further research.
Other diagnostic / screening tools
- Diagnostic examination of blood serum (serology) – Indirect sandwich ELISA (Enyme Linked Immunosorbent Assay; a technique to measure the presence of antibodies) is available but, as yet, not sufficiently reliable as a sole diagnostic tool due its potential to produce false positives. Currently this is not an effective screening test.
- PCR – Several substrates have been trialed including boot and cloacal swabs, but dust appears to be the substrate that is most suitable for a house diagnosis. Blackhead PCR kits vary in their sensitivity and specificity and the kit selected should be chosen following the most up to date scientific advice available. Flocks confirmed positive for blackhead with autopsy and histology will nearly always have a positive PCR result. However, positive PCR results are also possible when there are no clinical signs in the current flock and this can make interpretation challenging. These positive PCR results may be false positives or cross reactions; frequently there is a good correlation with previous blackhead outbreaks on the farm despite current flocks being clinically normal.
Note: PCR does not indicate presence of viable Histomonas but rather presence of Histomonas DNA.
Control and prevention
Due to the limited number of drugs available for treating blackhead prevention is key.
- Biosecurity. Good biosecurity between and within sheds is paramount. Outside clothing and footwear should be completely changed before entering poultry sheds; it is recommended that footwear is changed before entering each house using a biosecurity barrier system (a physical barrier prior to entry to the shed between which footwear must be changed). Movement of equipment between houses and on and off farms should be considered carefully and avoided if possible. Any equipment that does need to be moved should be thoroughly cleaned and disinfected. In areas with gallinaceous wild birds in the farm environment the risk is likely to be higher.
- If blackhead is diagnosed in one shed, then good between shed biosecurity will help to prevent the disease from spreading to other sheds. Increasing the temperature in the shed to dry the litter may also reduce the survival of the Histomonads in the litter.
- Litter removal. The complete removal of litter between flocks is recommended, especially after an outbreak and appropriate cleanout and disinfection procedures should be in place. Any sheds affected by blackhead should undergo a thorough and detailed cleanout and disinfection, possibly requiring extended cleanout time, and including the use of disinfectant products particularly effective against cecal worm eggs like sodium hypochlorite, quaternary ammonia and phenols. Litter should be removed from the sheds with as little contamination of the area outside the sheds as possible. Flaming and filling in cracks, together with removing dust from all areas of farm (not just sheds), may also help to reduce infection pressure.
- Cleanout protocols which include deep cleaning with industrial sweepers, the use of iodine, lime and also salting floors (granular feed grade only) in addition to using new litter have proven essential and successful even for dirt floors under field conditions. However, caution is advised, as salt has the potential to rust metal.
- Reducing primary disease carriers/intermediate hosts (i.e. cecal worms). This is one of the key steps in the strategy for blackhead control. Consistent, early, and scheduled worming following veterinarian advice will help reduce exposure to cecal worms/eggs and the Histomonads they carry.
- Treating for more than one day (3-5 days) has been shown to be more effective in some situations.
- There is evidence that using the same wormer for each treatment can lead to the development of resistance. To avoid this product rotation is recommended; avoid using the same product for more than three consecutive wormings.
- Vermin control. Consistent and effective rodent and insect control should be a core part of the biosecurity strategy for the farm. Besides cecal worms and earthworms, other organisms could serve as mechanical disease carriers. Therefore, control measures should also include actions to reduce darkling beetles, flies, and rodents among others. Measures should be taken to minimise the possibility of flooding as this will increase the presence of earthworms.
If flooding does occur disinfection of the flooded area must take place due to the potential increased presence of earthworms in dirt floor houses.
- Coccidiosis caused by E. tenella has been identified as an aggravating factor for blackhead.
- Blackhead is more likely to spread to the liver when coccidiosis is also present.
- The number of birds with severe lesions is increased when both Histomonads and E. tenella are present in the bird. As such, strategies to keep E. tenella under control are important.
- In many instances, there have also been blackhead cases identified right after E. necatrix, E. brunetti and E. maxima active infections.
- Vitamin supplementation, especially with fat-soluble vitamins A, E, D3, and K is a good practice. Absorption of fat soluble vitamins is decreased when intestinal diseases, such as blackhead occur.
- Good intestinal health. With the withdrawal of effective treatments, there is increasing interest in the development and use of alternative intestinal health products to mitigate blackhead disease issues. Nutritional products include: prebiotics, probiotics, organic acids, plant extracts, essential oils, enzymes, and volatile fatty acids, among others.
- Currently these products are supported by limited research, with results on their effects on blackhead being conflicting. For example, oregano-based products are reputed to have some limiting effect on the impact of blackhead but it has been difficult to prove this in the field.
- Control of E. coli. Although H. meleagridis is described as the causative agent of blackhead, it has been demonstrated that the parasite fails to cause clinical disease in the absence of bacteria. When E. coli and H. meleagridis were given to turkeys with no other bacteria present, the disease manifested. Several control strategies for E. coli had been used. The use of live and killed E. coli vaccines, organic acids via feed or water and the use of yeast cell based products that trap the bacteria and minimise their replication at the ceca, appear to decrease the severity of blackhead and may be helpful in the face of an outbreak.
- Vaccination. Histomonas vaccination based upon an attenuated clonal strain of H. meleagridis has been shown to be highly effective in experimental trials. However, further efforts are needed to standardise the production and optimise the administration of the vaccine in the field, and currently there is no commercially available vaccine.
Conclusions
Histomoniasis (blackhead) is a complex disease and much is still to be learned about the parasite that causes it. Introduction into a flock can occur in different ways and transmission between birds can occur with or without a disease carrier (vector; the role of cyst like stages still needs to be determined).
Since there are no available drugs to treat the disease, a multi-factorial approach is required to try and reduce or eliminate blackhead from an affected farm and prevent the problem including:
1. Improving biosecurity and having suitable cleanout procedures in place is essential.
2. Reducing buildup of cecal worms as the intermediate host and other potential vectors, e.g.,
- have an strong insect and rodent control program in place.
- deworm birds regularly (have a deworming protocol in place).
- thoroughly clean, disinfect and change litter after an outbreak.
- consider the use of specific floor treatment protocols.
3. The use of prebiotics, probiotics, phytogenics, organic acids and E. coli vaccines appear to help decrease the presentation of the clinical disease.
4. Having a plan of action with the local or company veterinarian to deal with the local situation and challenges is always good.