



Ingredient Nutrient Uplift By Enzyme Supplementation
Until more information is available, matrix values and uplift should be focused on the specific substrates for specific enzymes, recommended Dr Nelson Ward of DSM Nutritional Products to the Carolina Poultry Nutrition Conference 2008Excessive feed costs have prompted many nutritionists to consider enzymes as a tool to lower inputs, with energy and P (phosphorus) being the two focal points. This coincides with tremendous advances in enzyme technology, protein engineering and fermentation that generate products with heightened efficacy. The large number of publications in recent years is a testament to the interest in enzymes for both development and application (Engster, 2008) because enzyme use can serve an economical function through improved utilisation of substrates in feeds.
The application of matrix values is warranted for enzymes in a manner no different than for corn, soybean meal (SBM) and other ingredients. Phytases are widely accepted to improve the utilisation of P from phytate, thus these products often carry a matrix to reflect the efficacy and inclusion rate in the feed. The NSP (non-starch polysaccharide) portion in plant ingredients is the target for the “NSP enzymes”, and while the relatively high energy availability for corn (around 85 per cent) has restricted the use of NSP enzymes in corn-based diets, this is quickly changing.
Alternatively, enzymes could be used to improve feed conversion, body weight or other performance variables, but feed represents two-thirds of production costs. Meat supply is plentiful at a time when feed costs are high; hence, prudence favours a reduction in feed costs for economy and therefore, a nutrient uplift or matrix is the preferred route in the application of enzymes.
These matrix values are generated from animal studies designed to determine the amount of P, ME (metabolisable energy) and other components that are positively affected by an enzyme. While feed prices drive the focus toward the use of matrix values, due diligence is required since an enzyme’s ability does not magically increase to reflect the crisis mode of today’s ingredient prices.
Phytase
Typical corn/SBM broiler diets with 3-5 per cent meat and bone meal contain 0.22 to 0.27 per cent phytate P. Thus, phytase provides considerable opportunity to increase P availability, being that phytase supplementation has traditionally focused on the replacement of about 0.1 per cent available P. However, the high cost of phosphate has pressured phytase levels upwards to replace 0.12 to 0.15 per cent P. Considerations to supplant 0.2 per cent P have met considerable resistance, partly because of insufficient supporting data, and partly because the cost of the level of phytase needed may exceed the P being replaced.
The P replacement value for fungal and bacterial phytases is basically linear up to about 0.1-0.12 per cent P, followed by a plateau response. Thus, if 1X phytase = 0.1 per cent P, then 2X phytase = approximately 0.12 to 0.15 per cent. Shirley and Edwards (2003) tested up to the equivalent of about 20 times phytase in a study in which broilers were fed corn/SBM diets. With non-linear regression analysis on log-transformed phytase levels, nitrogen retention, feed to gain ratio (F/G), and apparent metabolisabl energy (AME) responded linearly, while all other variables (including tibia ash) responded quadratically. Tibia ash is considered the most sensitive variable on which to base the P replacement value for phytase, but weight gain, F/G and other attributes can be used.
A number of studies have been reported an improved trace mineral utilization when feeding phytase but the variation can be considerable. Until recently, cost of trace mineral supplementation has been low, such that savings from a trace mineral matrix value would have been minimal from a least-cost standpoint.
ME matrix values for phytase products have a considerable range, largely due to interpretation or the philosophy of the supplier. For fungal phytases, ME recommendations are from about 5 to 25 kcal/lb (pound) final feed. For bacterial phytases, the disparity ranges from one commercial bacterial phytase with 25 kcal ME/lb final feed, while another’s recommendation has no increase in ME.
An improved ME from phytase should be a direct correlation with phytate degradation by phytase. Thus, if two phytases at a given amount result in 0.1 per cent P, than that given amount of phytase should impact ME equally for both phytases. Phytate is the antagonist and once removed to the equivalent of 0.1 per cent P – regardless of the form of phytase – the resulting improvement in ME logically would be the same.
The author's experience with different fungal and bacterial phytases indicates a fairly low ME value can be gained, regardless of the phytase. An addition level of 1X or 2X (with 1X = 0.1 per cent P) has given no significant difference in ME value across fungal and bacterial phytases. Owing to the wide disparity in philosophies to assign commercial values across companies, matrix values for ME and amino acids have virtually no value when evaluating commercial phytases.
In addition, the evaluation of phytase should focus on the cost to replace a given amount of P “per ton feed”, since considerable modifications in phytase analyses make the use of units (specifically FTU) for comparison purposes especially confusing (Ward and Campbell, 2007).
Crop activity
Most of the phytase activity and phytate breakdown occurs in the crop, proventriculus and gizzard, with minimal activity in the small intestine. Liebert et al. (1993) noted about 50 per cent or more of the activity of fungal A. niger occurred in the crop.
Considerable phytate hydrolysis occurs in the crop for P. lycii fungal phytase, as opposed to two different bacterial E. coli phytases (Glitso et al., 2007; Sorenson et al., 2005). Both phytases were added separately to a corn/SBM feed to replace approximately 0.1 per cent P. The crop contents were taken to determine phytate disappearance for the three groups: control (no phytase), fungal P. lycii and bacterial E. coli.
With E. coli phytase, 37 per cent of the phytate was degraded, whereas the P. lycii group experienced a decrease of 78 per cent (Figure 3). In other words, the P. lycii appears to be doing most phytate hydrolysis in the crop.
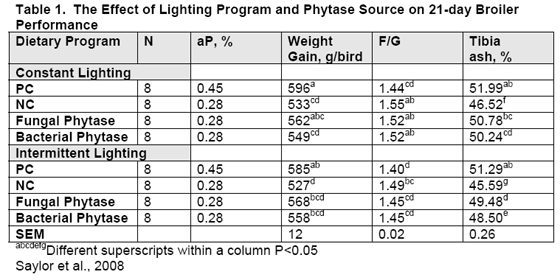
Based on the ability of P. lycii to release P in the crop, the University of Delaware and University of Maryland tested the effect of lighting on phytase efficacy (Saylor et al, 2008), since lighting programmes are known to stimulate meal feeding (crop fill). One programme was designed to encourage crop fill and the other was the 21L:3D traditionally used with most battery studies. P. lycii and an E. coli phytase were supplemented to the feed to replace approximately 0.1 per cent P as separate treatments, and feed levels were confirmed by analysis.
Overall, as compared to the constant lighting, the chicks on the intermittent lighting performed with increased (P<0.05) efficiency, no difference in body weight, a 16 per cent lower feed consumption and a lower (P<0.05) tibia ash.
With the intermittent programme, a significant (P<0.05) increase occurred in 21-day tibia ash of P. lycii birds over those fed E. coli (Table 1). Body weights and F/G did not differ between phytase sources. The lighting experiment agrees with the study which found P. lycii to accomplish more phytate degradation in the crop than did the bacterial phytase. Commercial conditions utilise some degree of intermittent lighting, all of which favour meal feeding (crop fill). Crop weight as a percentage of body weight was greater (P<0.002) for birds with intermittent lighting as opposed to 23 hours light recently (Dalal et al., 2008). Comparative trials with all-light programmes may not allow for these differences to be expressed, yet under commercial conditions, such difference may become more evident since intermittent lighting are common commercially.
NSP Enzymes
More wheat than normal has been used in the US during this calendar year, for which xylanase was used in the diets to avoid wet litter conditions, lowered feed intake and other performance-related problems related to soluble NSP. Amylase enzymes can also elicit improvements with such ingredients. Depending on the level of wheat and xylanase fed, and the type of wheat, an ME matrix value of up to 30 to 40 kcal/lb final feed is not uncommon. Amino acid responses have also been attributed to xyalanses in wheat-based diets, thus sometimes have been included in a matrix.
While they lack the viscous nature of NSPs found in wheat, barley and rye, NSP in non-viscous cereal grains pose a physical barrier between the intestinal enzymes and cell components (Hesselman and Aman, 1986). In doing so, starch, protein, oil and other nutrients are encapsulated within the plant cell. Energy gained from the complete NSP digestion of the cell walls is insignificant. Instead, the greatest nutritional value is expected from the released components inside the cell. Some evidence suggests that NSP can stimulate mucin secretion through an increase in goblet cells (Satchithanandam et al., 1990), and possibly disrupt and hinder normal digestive processes. Choct (2001) outlines other detrimental aspects of insoluble NSP.
Corn
For the most part, however, the insoluble NSP portion of ingredients presents the greatest challenge in most US diets. For corn grain, this would be mainly the arabinoxylans and cellulose. The author recently analyzed two groups of US corn grain (n=23). The arabinoxylan content was close to four per cent, while the cellulose fell within a range of two to four per cent. These two components, along with about one per cent pectins, comprise 90 per cent of the NSP in corn, or 9 to 10 per cent of the dry matter (Malathi and Devegowda, 2001).
SBM
The oligosaccharides and polysaccharides are of concern in SBM due to their indigestible nature and level (18-21 per cent; Bach Knudsen, 2001). The oligosaccharides are mainly α-galactosides (raffinose and stachyose) and are not digested by endogenous enzymes. These make up six per cent of the SBM dry matter, and are associated with wet litter due to bacterial degradation in the lower intestinal tract. The ratio of ME to gross energy in SBM is 0.51 for poultry (NRC, 1982), indicating about 51 per cent of the gross energy in SBM is used for metabolic functions. The removal of oligosaccharides with ethanol resulted in 10 to 15 per cent or more improvement in TME (Coon et al., 1990).
Corn DDGS
In a group of 30 samples from throughout the US, the total NSP content of corn DDGS was 23.1 per cent of the dry matter, and the insoluble NSP comprised 88 per cent of this (Ward et al., 2008). The arabinoxylan and cellulose accounted for 85 per cent of the NSP in corn DDGS.
Thus, due to the complexity of NSP in typical corn/SBM diets, a multiple-enzyme addition would likely be the most beneficial for an energy matrix value or uplift. For corn grain and corn DDGS, xylanases and cellulases utilise the arabinoxylans and cellulose as substrates, while pectinases, mannanase and galactosidases target the pectins in SBM. Malathi and Devegowda (2001) reported that groups of enzymes high in pectinases released more sugars in SBM than other combinations tested.
Amino Acid Effect
Through its degradation of phytate, phytase can permit a greater utilisation of proteins (Ward 2006). A reduction in phytate may also reduce excessive mucin secretion, thus could make the animal more “nutrient efficient”. A number of researchers have reported beneficial effects on amino acid digestibility (Zanella et al., 1999; Saleh et al., 2005; Meng and Slominski, 2005). In one study, the apparent ileal digestibility of amino acids was significantly higher in the corn/SBM diets supplemented with a combination of endoxylanase, α-amylase and ß-glucanase (Rutherfurd et al., 2007). Overall, amino acid digestibility was improved by more than five per cent. In the absence of a supplemental protease, this increase is attributed to the degradation of the fibre matrix surrounding the protein, thus allowing intestinal proteases greater access. Enzyme supplementation did not influence ileal endogenous amino acid loss, evidence that the improved amino acid digestibility occurred by the actual breakdown of protein by proteases already present.
Across trials, carbohydrases in corn/SBM diets generally show a two to three per cent improvement for amino acids. Some products may include “uplift” for amino acids but ME historically has been the focus for carbohydrases. These products recommend in the vicinity of 30 kcal/lb final feed, depending on inclusion rates and other aspects.
Synergism, Additivity and Antagonism
For the most part, there is little evidence that phytase + carbohydrases work synergistically, i.e. the final outcome is greater than the sum of the parts; 1+1>2) across all diets. Theoretically, and considering the binding of phytate within the fibrous matrix of some ingredients, one could expect carbohydrases to make phytate more available to phytase, thus work synergistically with the carbohydrases. This is more apt when NSP content is higher than in corn/SBM diets, or when viscosity is an issue for wheat, rye or barley diets.
An additive effect is more likely between two unrelated enzymes (the final outcome is equal to the sum of the parts, i.e. 1+1=2). Questions exist as to whether we truly see this; some studies are confounded without suitable negative controls and treatments. The simple addition of the ME value for phytase and the ME for carbohydrase products can be a “stretch”, especially if one or the other is already excessive. Between phytase and carbohydrases, the latter would have the greater potential for improving ME in most diets, regardless of the ingredients.
One would not necessarily expect antagonism (final outcome is less than the sum of the parts; i.e. 1+1<2) but this aspect has not been completely studied. The author has recently concluded work which finds protease + phytase to work effectively with no obvious antagonism of the protease on phytase function. Other proteases likely need similar confirmation.
Currently, a prudent position seems in order when adding all enzymes to the same feed: phytases for P, calcium; carbohydrases for ME; proteases for amino acids. By no means, however, is the issue resolved as to how various enzymes work together, and how the “uplift” or matrix will be ultimately be modeled.
Parting Comments
The use of enzymes in poultry diets is widespread, owed largely to the cost of ingredients. Yet, without advances in the technology of enzymology, protein engineering and fermentation, the extensive use of enzymes would not be feasible. Practical use of enzymes by nutritionists usually includes matrix values focused on those nutrients or components that act as substrates. The carbohydrases offer a grand opportunity for enhancing economic value of ingredients, with ME being the primary focus for corn/SBM type diets. Amino acid nutrition may be improved by carbohydrases, although not always, but this is generally viewed as a secondary effect through the breakdown of the various complex carbohydrates. Strong support for synergism across enzymes is lacking, and additive effects are not always obvious. Until resolved, matrix values and uplift should be focused on the specific substrates for specific enzymes.
Literature Cited
Dalal, S., U. Fernando, K. Schwean-Lardneri, B. Laarveld, H.L. Classen, A. Kessel and B. Fancher, 2008. Effect of photoperiod on upper gastrointestinal tract microbial ecology in broiler chickens. Poultry Sci. Mtgs, Niagara Falls.
Engster, H., 2008. Rising feed costs and potential impact on broiler health and performance. 43rd National Mtg Poultry Health Processing, 25-32.
Bach Knudsen, 2001. The nutritional significance of “dietary fibre” analysis. Anim. Feed Sc. Tech. 90:3-20.
Choct, M., 2001. Enzyme supplementation of poultry diets based on viscous cereals. In M.R. Bedford and G.G. Partridge, Enzymes in Farm Animal Nutrition. CABI Publ., New York, NY.
Coon, C.N., K.L. Leske, O. Akavanichan and T.K. Cheng, 1990. Effect of oligosaccharides on true metabolizable energy and fibre digestion in adult rooster. Poultry Sci. 69:78.
Hesselmann, K. and P. Aman, 1986. The effect of ß-glucanase on the utilization of starch and nitrogen by broiler chickens fed on low- and high-viscosity barley. Anim. Feed Sc and Tech. 15:83-93.
Malathi, V., and G. Devegowda, 2001. In Vitro Evaluation of Nonstarch Polysaccharide Digestibility of Feed Ingredients by Enzymes. Poultry Sc. 80:302-305.
Meng, X., and A. Slomenski, 2005. Nutritive value of corn, soyabean meal, canola meal, and peas for broiler chickens as affected by a multicarbohydrase preparation of cell wall degrading enzymes. Poultry Sc. 84:1242-1251.
Rutherfurd, S.M., T.K. Chung and P.J. Moughan, 2007. The effect of a commercial enzyme preparation on apparent metabolizable energy, the true ileal amino acid digestibility, and endogenous ileal lysine losses in broiler chickens. Poultry Sc. 86:665-672.
Saleh, F., A. Ohtsuka and K. Hayashi, 2005. Effect of dietary enzymes on the ileal digestibility and abdominal fat content in broilers. Animal Sc. J. 76:475-478.
Satchithanandam, S., M. Vargofcak-Apker, R.J. Calvert, A.R. Leeds and M.M. Cassidy, 1990. Alteration of gastrointestinal mucin by fibre feeding in rats. J. Nutr. 120:1179-1184.
Saylor, W., N.E. Ward and R. Angel, 2008. Intermittent lighting affects phytases differently. Final report.
Shirley, R.B. and H.M. Edwards, 2003. Graded levels of phytase past industry standards improves broiler performance. Poultry Sci. 82:671-680.
Ward, N.E., 2006. Phytase matrix values: theoretical aspects. Feedstuffs Vol 78, No 4, January 23.
Ward, N.E., and D. Campbell, 2007. Phytase assessment requires understanding. Feedstuffs, Vol 79, No. 22, May 28.
Ward, N.E., R.T. Ziljstra, C. Parsons and C. Starkey, 2008. Non-starch polysaccharide (NSP) content of U.S. commercial corn distiller’s dried grains with solubles. Abstract, SPSS, Atlanta GA.
Zanella, I., N.K. Sakomura, F.G. Silversides, and A. Fiqueirdo, 1999. Effect of enzyme supplementation of broiler diets based on corn and soybeans. Poultry Sc. 78:561-568.
Further Reading
| - | You can view other papers presented at the Carolina Poultry Nutrition Conference 2008 by clicking here. |
May 2009








