Key points of biosecurity for Gumboro Disease control
Six key biosecurity points need to be successfully managed to control IBD appropriately.Infectious bursal disease (IBD) is an acute viral infection for young chicken (broiler and pullets) caused by the IBD virus (IBDV), classified in the Birnaviridae family, genus Avibirnavirus (Eterradossi and Saif 2020). This virus is non-enveloped, with 2 segments of RNA and a very resistant capsid, making it a resident pathogen in a farm. Cleaning and disinfection are key to decrease the IBDV pressure and to stop the Gumboro cycle. This procedure needs to be associated with a strict farm isolation and an adapted vaccination program which protects the immune system from infection.
Six key biosecurity points need to be successfully managed to control IBD appropriately.
1. How to create a clean area:
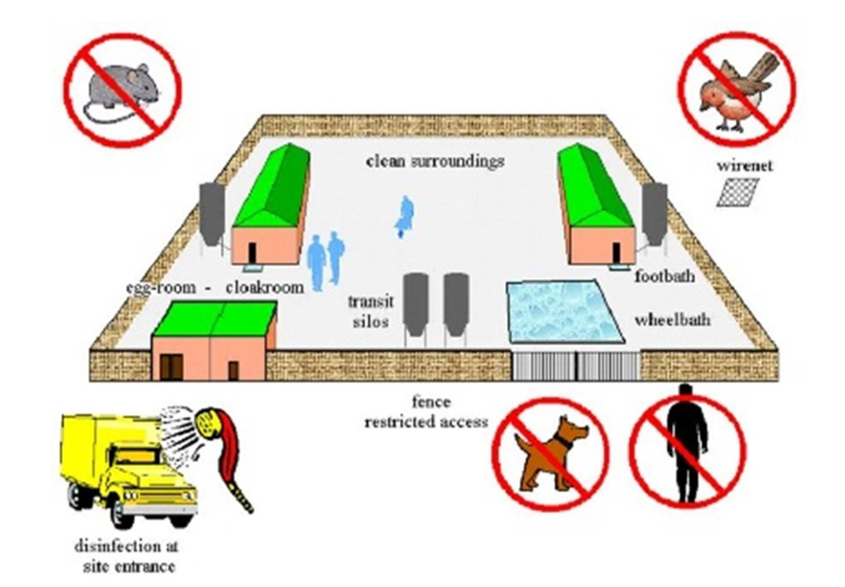
Around the farm, walls or wired fences separate external and internal farm areas, thus creating an epidemiological unit. This unit must be isolated and secured (fig. 1). The employees and visitors must take a shower and use farm-specific PPE (boots, overall, gloves, mask, hairnet) to work inside the farm. For IBDV, the higher risk of external contamination are hands, boots, and dust around any house entrance (e.g. gates at the house front for day-old chicks and litter placement and doors at both sides to access the equipment and control room). The area around the cooling pad area is also critical for dust accumulation (fig 2). The equipment and control room of each house must be divided into 2 zones, each zone using its own pair of boots, identified with different colours (outer area and inner area).
2. How to avoid vectors entering the farm/house:
Rodents and insects (darkling beetles, flies) are considered a dynamic vector of IBDV, since they move around different farms & houses, are a potential reservoir of IBDV in the house in turnaround times.
Rodent’s control: Rodents can be present anywhere. Set a bait with rodenticide every 10 meters around the house, storage room, office, etc. Record the results (bait consumed or unconsumed) and change the rodenticide bait every month. It should contain difenacoum or brodifacum as active ingredient. All feed residues must be thoroughly cleaned, especially under the feed silos.
Flies’ control: Flies like the smell of feed and color (red, yellow) (fig. 3). To capture adult flies, use insecticides (thiamethoxam, permethrin or neonicotinoid) and sex pheromone baits (tricosene as example) + sugar. Mixing 5 g. of insecticide with 3 water droplets in a recipient is enough to control flies in 50m². To identify wet aera (litter under water pipeline, floor in the corner must be dry.
Darkling beetles’ control: once the very last broiler leaves the house, the darkling beetles hide quickly in wall cracks or areas with hard access (fig. 4). You have only a few minutes to spray a concentrated solution of insecticide containing pyrethroids (tetramethrin), pseudo pyrethroids (Etofenprox), Nicotinoids (Acetamiprid) or spinosad on 50 centimeters around the wall-floor junctures. Cracks and holes in the floor and the walls must be filled. Around 80 L. of solution is enough to spray a floor perimeter of 1000 m², for example.
3. Litter management:
1 gr. of used litter might contain up to 106 viruses. At clean-out, all dust resulting from litter removal must be cleaned up to greatly decrease viral challenge. Ideally, use conveyors tunnels made of plexiglass directed to trucks with a trailer cover. Be careful with the wind currents. The floor must be cleaned with automatic sweeper (if available) and manually (picture n°4). For dirt floor, quicklime powder from the previous cycle would help while cleaning up litter residues.
4: Cleaning and disinfection protocols (all surfaces, including minor equipment):
The objective of a cleaning compound is to reduce the amount of biofilm on the surface. Acid cleaners are used against mineral materials and enzymatic cleaners against organic material; however, basic cleaners are most effectively sprayed with a foam-gun (fig. 5). After 30 minutes of contact time, the contact surfaces must be rinsed with high pressure water.
Applying the disinfectant: One must calculate the developable surface by adding up all the elements inside the space we need to disinfect.
E.g.: Developable surface (m2) = floor + wall + roof + equipment + cooling pad + fan + pipeline + feed line + plates.
This calculation might vary, depending on which material the house is made of or contains (concrete, slate floors, or cages). The developable surface would then vary accordingly, between 3-44 times more than a regular concrete floor house (this coefficient calculation is available, ask our local team).

Dosage: Disinfectant suppliers generally describe the % of disinfectant needed to inactivate a virus (using specific virucidal efficacy tests during conformance processes). If the IBD virus activity is 1%, we should spray 3 ml. of disinfectant per m². Multiply this by the amount of developable surface (calculated above) and you will obtain the required amount of disinfectant needed. Depending on the surface’s liquid retention capacity, you might need to estimate the quantity of water required to humidify all surfaces, to not get short of solution.
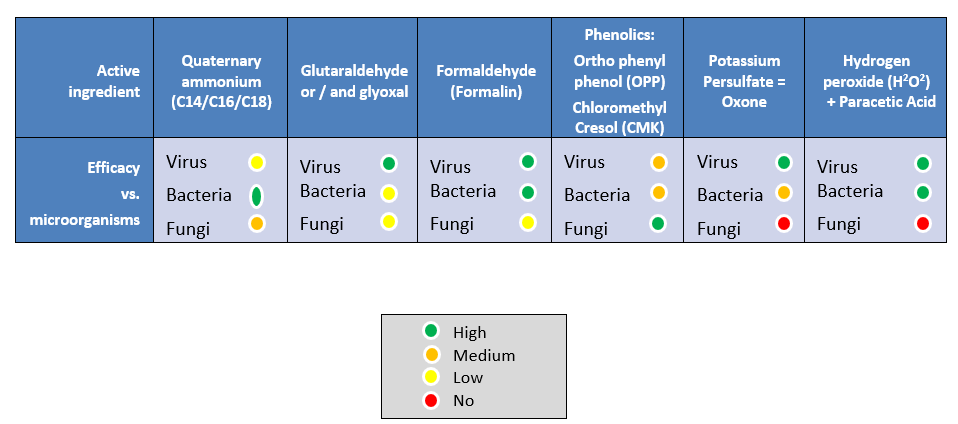
Disinfectant choice: Glutaraldehyde-quaternary ammonia; oxidizers, phenolics are effective if the contact time is enough and an appropriate quantity of disinfectant is sprayed on all surfaces. It is important to make a proper cleaning and disinfection of the wall-base because this part of the wall is in direct contact with day-old chicks and litter residues might remain uncleaned. Disinfectant activity reduces 5 logs of IBDV each 30 minutes.
Minor equipment could be cleaned and disinfectedcted in a specif a specific(outside the house) using a specific protocol. Firstly, remove the organic material attached by brushing it and place it in a cleaner tank. After 30 minutes of contact time, rinse it and add the disinfectant solution. Let it dry out on a specific area.
5: Floor disinfection (e.g. house 1000 m²)
A sodium hydroxide solution (100 kg. / 500 L. water) can be applied on the floor, expecting a 2-15 mm. of penetration within the material. After 6 hours, apply 250 kg. of quicklime powder (doubling the dose for earthen floor) and spray water for activation (200-600 L.)
Down time period definition: Time spent between cleaning and disinfected of all surfaces and the new litter and the equipment is placed again. An effective downtime period could be maximally reduced to a few days (2-4 days, to be sure that all surfaces are dry).
Specific case of the slat: To optimize efficiency of slat disinfection, the slat are disinfected outside in a specific area (as small equipment).
Protocol:
- seaking in water tank
- brushing
- cleaner tank with detergent
- contact time area 30minutes
- rincing;
- disinfectant tank
- drying area + storage.
6. Litter placement (or slat positioning).
The litter (with good quality raw material: no dust) reduces the risk of contact between the DOC’s and the floor (considered the major virus reservoir). A thickness of 10 cm. of litter is recommended for good protection, liquid retention, and to avoid fermentation. Litter turning could be dangerous because the virus might then be exposed to the surface and come in close contact with the birds.
Conclusion:
A proper cleaning and disinfection protocol is essential to decrease the IBDV load during the downtime period and just before new placement of day-old chicks. The efficiency of this protocol could be challenged by wasp (resistant tissu cloth) and IBDV qPCR 2 days after disinfection. Disinfectant neutralizers must be used during sampling and at the laboratory to minimize false negative results.
A secondary fogging disinfection after litter placement is optional to secure disinfection (interesting in cage or slat production) but it is recommended when IBDv pressure is high for example in old houses or in big complexes.