



Newcastle disease control: LaSota or cloned vaccines, looking for the best option
Introduction
Newcastle disease is a challenge worldwide, causing economic losses because of high levels of mortality, a drop in production and economic restrictions. It is endemic in Latin America, Asia, Africa and parts of Europe. It is regarded as exotic in a few countries such as the United States, Brazil and parts of Western Europe.
It is caused by a Paramixovirus serotype 1 (APMV-1), the APMV-1 is classified into two groups, Class I and Class II. Class I has just one genotype, was isolated in wild birds and is asymptomatic. Class II contains at least 18 genotypes (I – XVIII) and in some cases these genotypes can be divided into subgroups. Genotype XII is divided into subgroups XIIa and XIIb).
According to the OIE, Newcastle disease is defined as an infection of birds caused by a virus of avian paramyxovirus serotype 1 (APMV-1) that meets one of the following criteria for virulence:
a) The virus has an intracerebral pathogenicity index (ICPI) in day-old chicks (Gallus Gallus) of 0.7 or greater
or
b) Multiple basic amino acids have been demonstrated in the virus (either directly or by deduction) at the C-terminus of the F2 protein and phenylalanine at residue 117, which is the N-terminus of the F1 protein. The term 'multiple basic amino acids' refers to at least three arginine or lysine residues between residues 113 to 116. Failure to demonstrate the characteristic pattern of amino acid residues as described above would require characterisation of the isolated virus by an ICPI test.
We have many options regarding live vaccines for the control of NDV field pressure, and for reducing clinical signs, but vets are often unsure about which options should they use and what pros and cons might be expected.
Epidemiology and pathogenesis
As with all pathologies affecting poultry, the first step should be decided on the basis of the disease itself, by knowing the pathology and epidemiology of the NDV in this case.
The two main reservoirs of the disease are backyard birds and wild birds (especially aquatic). The migration routes of wild birds have the potential to spread the virus.
Transmission is horizontal and depends on the organs in which the virus multiplies. Birds with respiratory disease spread the virus mainly through the air by aerosols from contaminated mucus, which may be inhaled by other susceptible birds, and in lower quantities by faeces.
Some NDV viruses show tropism to intestinal replication, although they are able to replicate in respiratory and reproductive tissues as well. These viruses are transmitted mainly by ingestion of contaminated faeces, contaminated feed or water, or by inhalation of small infective particles produced from dried faeces.
The disease is spread by fomites, people and poultry products from contaminated poultry farms, including dead birds and natural fertilizer from poultry litter farms. The NDV is able to survive in the carcases or excretions on the litter for several weeks at cool ambient temperatures or for several years if kept at frozen temperatures.
The virus is shed from the very early stage of infection, during the clinical stages and for a limited period during convalescence.
The period between infection and the appearance of clinical signs varies from 2 to 15 days, for the purposes of the OIE Terrestrial Animal Health Code, it is 21 days.
The basics of the control
The vaccination programme is one of the tools to control the clinical signs of the disease and reduce virus shedding. The most usual vaccination programmes against NDV are based on live and killed vaccines (mainly in long-life birds) and with a combination of just live vaccines, live + killed vaccines or live + vectored vaccines.
Live vaccines will provide local immunity (cell-mediated immunity and local humoral immunity) a few days after vaccination, which is necessary to protect the mucosa, and humoral systemic immunity after 2 weeks.
Inactivated vaccines will provide greater and more stable humoral immunity but they do not develop a strong cell-mediated response.
The best route of vaccination with live vaccines against Newcastle disease is with the use of the main route of infection (respiratory), because of the limitations of the drinking water vaccination method (water temperature, chemicals and time of vaccination). The vaccines with respiratory tropism are the LaSota strain-based vaccines or B1 strain vaccines.
Depending on the field pressure challenge, we should adapt our vaccination programme to the different kinds of products that we can find on the market. As an example, we could say that B1 type vaccines are mainly used as priming at the hatchery, whilst LaSota and cloned vaccines are mainly used for revaccination on the farm.
Why do we use different strains for different purposes?
Mainly because vaccines with better efficacy performance (LaSota and cloned) may show some post-vaccine reaction when they are not used properly, whilst B1 will show a better performance in terms of safety, but lower efficacy when compared with LaSota and cloned vaccines.
Between LaSota and cloned vaccines, we should bear in mind that finally, a product such as Hipraviar® CLON is a LaSota strain, but cloned. This means that through the cloning procedure we look for the best option from a parent LaSota virus, we select a virus population with increased efficacy and reduced post-vaccine reactions, so we could call it an “improved” LaSota strain. However, what really makes the difference is how the lab selected the correct cloned strain in order to maintain LaSota efficacy.
In a recent study conducted in the Laboratory of Avian Pathology of the Faculty of Veterinary Medicine, National University of San Marcos in Peru, 90 birds were used which were assigned to three treatment groups of 30 birds each:
- Group T2 received HIPRAVIAR® CLON (a cloned LaSota vaccine, strain CL79) at 2 and 11 weeks of age and LaSota conventional vaccine (HIPRAVIAR® S) in the eighth week of life.
- Group T3 received HIPRAVIAR® CLON/H120 (a cloned LaSota vaccine, strain CL79) at 2, 8 and 11 weeks of age.
- Group T4 received a competitor´s cloned LaSota vaccine at 2, 8 and 11 weeks of age.
All the groups received polyvalent inactivated vaccines from the same lab at 3 and 14 weeks of age.
All the birds were challenged at 27 weeks of age with 0.1mL of an inoculum containing a vvENC GXII strain LD50 and titre 106 EID50, intramuscularly and with an intracranial pathogenicity index (ICPI) of 1.88. The variables that were evaluated daily were:
- Percentage of cumulative egg production and egg quality, during the two weeks post infection.
- Eggshell quality
Results
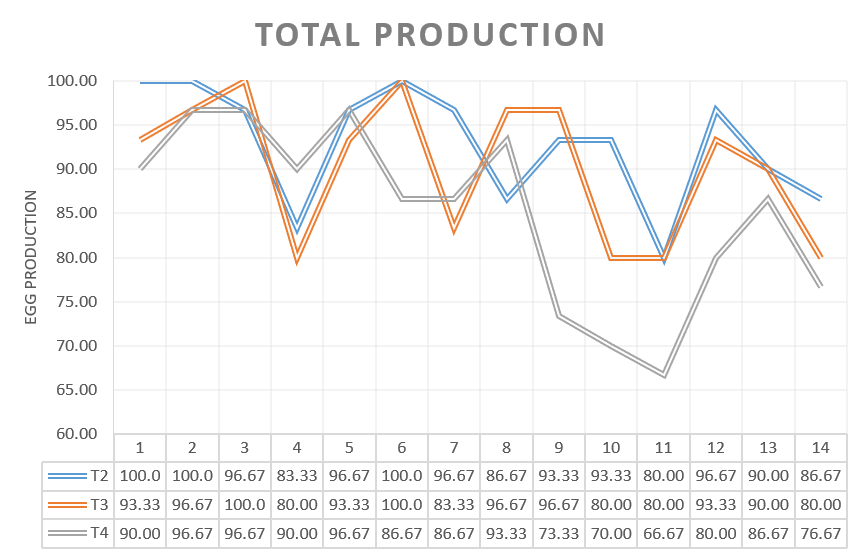
The percentage of egg production during the 14 days was analysed for the three groups challenged in production.
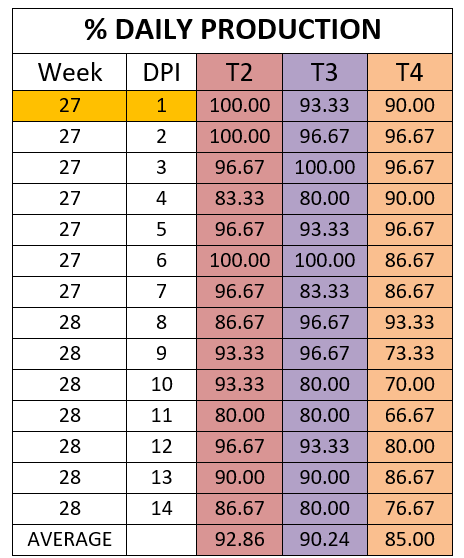
The percentage of eggs with low shell quality was 10.24 percent, 8.81 percent, and 13.57 percent for groups T2, T3 and T4 respectively.
Conclusions
All the vaccination programmes protected the birds from mortality and respiratory or nervous clinical signs.
Egg production was affected in the 3 experimental groups as well as eggshell quality, however, the percentages of decreased egg production and shell quality defects differed between groups. The group vaccinated only with HIPRAVIAR® CLON/H120 showed the best results for percentage of eggs with shell defects whilst in terms of egg production it was similar to T2, vaccinated with cloned and Lasota strains. T2 and T3 showed better performances in terms of egg production and eggshell quality when compared with the results for group T4, which was vaccinated with the competitor´s cloned vaccine.
The greatest decrease in production was observed in the week following the challenge and the highest percentage of low quality eggs was observed during the same period.
The results of the study allow us to conclude that all groups showed very good protection against local respiratory problems and group T3 (vaccinated with HIPRAVIAR® CLON/H120) showed the same efficacy as a group where LaSota strains were used. On the other hand, group T4 (vaccinated with a competitor´s cloned vaccine) showed lower egg production and a higher percent of shell defects than groups T2 and T3.
We can conclude that each cloned vaccine should be considered individually, as the selection process seems to be very important in order to keep LaSota´s efficacy and reduce post vaccine reactions. Another key point to be assessed with vaccines against the NDV is virus titre, as has been highlighted several times (Cornax, I., Miller, P.J. and Afonso, C.L. 2012),
All Hipra´s live vaccines against ND are always formulated below a minimum titre of 10⁶,⁵ EID50, providing an optimal balance between efficacy and safety, and with a wide range of products ready to be used under all circumstances.
It is clear that the most logical choice is the HIPRAVIAR® CLON and HIPRAVIAR® CLON/H120 (strain CL79).
CL79 strain is a cloned LaSota strain, designed to give a lower reaction compared to the conventional LaSota, with an IPIC of 0.15, and the same efficacy, as per the trial above.
More HIPRAVIAR®
CLON and HIPRAVIAR® CLON/H120 trials like this one
| References | ||||
|---|---|---|---|---|
| Orsi MA, Doretto Júnior L, Reischak D, da Silva LHA, Spilki FR, Buzinaro MG, Arns CW | ||||
| Newcastle Disease Virus Vaccine Strains: Immunogenicity is not Influenced by ICPI.. Brazilian Journal of Poultry Science | ||||
| Cornax, I., Miller, P.J. and Afonso, C.L. | ||||
| (2012) | Characterization of Live LaSota Vaccine Strain–Induced Protection in Chickens upon Early Challenge with a Virulent Newcastle Disease Virus of Heterologous Genotype, genotypes III, VIg, VIb, VIId, and IX.. Avian diseases | 56:464–470. | ||
| Calnek, B.W, Barnes H. J, Beard C. W, Mcdougald L. R, Saif Y. M, | ||||
| (2000) | . Disease of Poultry. | Cap 20. 2nd edtion. 567 pp. | ||
| Hy-Line International. | ||||
| (2018) | Hy-Line variety Brown, commercial management guide.. Hy-Line International. Iowa, United States. | 41 pp. | ||
| Alexander, D. | ||||
| (2003) | Newcastle Disease and Other Avian Paramixoviridae Infections.. In: Diseases of Poultry, Cap 2. 11th Edition. | Saif, Barnes, Glisson, Fadly, Mc Dougald, Swayne (eds.). AAAP Iowa State University - USA. 63-87. | ||
| ICTV | ||||
| (2019) | International committee on taxonomy of viruses. In: Virus Taxonomy: 2018b Release | |||
| Miller P.J and Koch G., | ||||
| (2013) | Newcastle Disease, Other Avian Paramyxoviruses, and Metapneumovirus Infections. Newcastle disease.. In: Diseases of Poultry, 13th Edition. Ed. | By Swayne, D.E., Glisson, J.R., Mc Dougald, L.R., Nolan, L.K., and D.L. Suarez. Edit. Willey-Blackwell Publishing. Iowa, USA p. 89-107. | ||
| Umali, D.V., Ito, H., Shirota, K., Ito, T., & Katoh, H. | ||||
| (2015) | Atypical velogenic Newcastle disease in a commercial layer flock in Japan.. Poultry Science | 94 (5), 890-897.doi: 10.3382 / ps / pev011 | ||
| Khorrajiya JH, Pandey S, Ghodasara PD, Joshi BP, Prajapati KS, Hodasara DJ, Mathakiya RA | ||||
| (2015) | Patho-epidemiological study on genotype-XIII Newcastle disease virus infection in commercial vaccinated layer farms.. Veterinary World 8 | (3): 372-381. |









