



Newcastle disease: Key points about vaccination
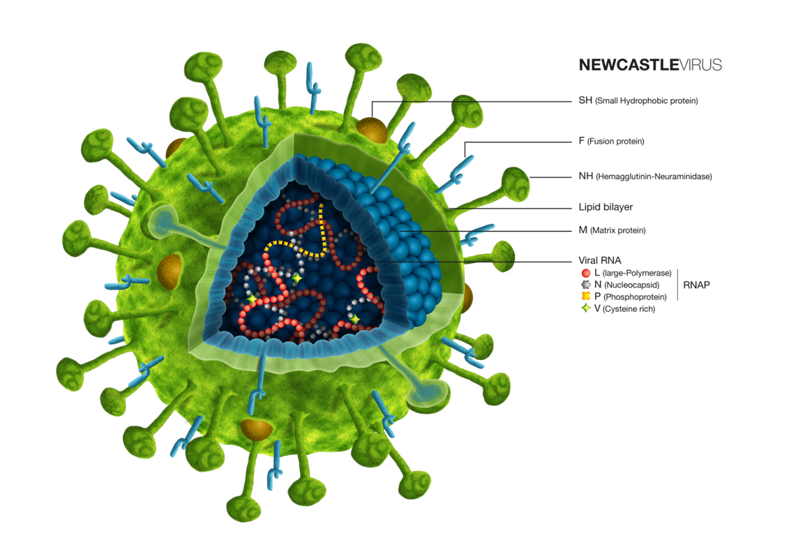
Newcastle disease is one of the most important diseases in poultry, present in almost every country in the globe, and is the cause of economic losses as a result of mortalities and trading embargos.Newcastle disease is caused by the Avian Paramyxovirus serotype 1. The virus has different serotypes, although only serotype one is the cause of the disease, and there are also different genotypes and sub genotypes within the same serotype.
The genotype classification is based on the partial sequencing of the F gene and the classification is divided into XVIII genotypes and sub genotypes, the most predominant genotype of Newcastle disease currently being genotype VII. The genotype difference does not affect immunity, but it is used to understand the evolution of the disease.
Throughout its history, Newcastle disease has become pandemic four times. The very first time was in the mid 1920´s and it occurred in the UK, in the city of Newcastle-upon-Tyne and in Java in Indonesia. The first of these gave the disease its name, which was supposed to be temporary, as the researchers at the time did not have confirmation that it was a new disease or just a different symptom of an already known disease. This first appearance took 20 years to become a pandemic and we are currently in the fourth pandemic. We identify the different pandemics because the predominant genotype of the virus will be different.
© HIPRA
The role of vaccination in immunity
Normally, vaccination programmes are divided between basic types of vaccines, live vaccines and inactivated vaccines. The live vaccines will provide early immunity due to the possibility of the chicks having early exposure to a field challenge shortly after vaccination, so this early protection is mostly related to cellular protection (cell-mediated immunity or CMI). Another important role of live vaccines is priming for inactivated vaccines, as an inactivated vaccine primed with a live vaccine will have higher antibody count.
Antibody levels are related more to the use of inactivated vaccines, but how they vary depends on the priming of the live vaccination (Icochea, 2019). The variation will depend on the strain of the live vaccine, the titres of the vaccine and the method of vaccination. As general rule, a cloned LaSota strain will provide good priming (as the LaSota strain can be challenged due to post vaccine reaction).
Higher vaccine titre is beneficial, because you will have protective titer despite the decrease of the titre because of the method of application and the vaccine handling, normally Newcastle vaccines have titres that are in the range of 10 5.5 to 10 6.5 (Tonya, 2017).
How to design a vaccination programme
In order to design a vaccination programme, it is important to consider:
- The birds to be vaccinated: Broiler Breeders, Layers and Broilers will differ mainly because of their age and in breeders it is important to consider the level of maternal antibodies for the progeny.
- The pressure of infection and the legal requirements in the country. It is important to analyse the local situation, for which it is important to check past laboratory reports (most importantly the serology). The historical serology will provide valuable information about the pressure of infection in the area and the ¨normal¨ baseline, the levels to be considered suspect and the levels to be considered high. The most widely used serological tests are ELISA (Enzyme-Linked Immunosorbent Assay) and HI (Hemagglutination Inhibition Assay), which each have their strengths and weaknesses. Whichever is chosen, the most important thing is to have historical data.
- The vaccine type and method of application:
- Regarding Inactivated Vaccines, it is important to consider the antigenic mass within the vaccine, in other terms the concentration of the vaccine. Inactivated vaccines are dose-dependent and the seroconversion after vaccination will be proportional to the applied dose and concentration, so that the application must be efficiently managed, especially in a low dose scenario, e.g. 0.1 ml.
- Vector Vaccines: Vector vaccines will provide seroconversion after 3 or 4 weeks from the time of application and in that regard, they will be similar to inactivated vaccines. In order to bridge the gap between full seroconversion and the vaccine application, a full live vaccination programme is required.
- Live vaccines, some points are important: the strain, related to the pressure of infection in the area. For example, in medium to high-risk areas, the use of a cloned LaSota strain is beneficial, because it will deliver the highly effective strain, namely the LaSota strain without the disadvantage of this strain, that is reactivity. Another important point is the titres, as the titres will help to control the disease. In the case of vaccines with higher titres, the decrease in those titres due to vaccination management will not compromise protection.

Conclusions
There cannot be a standard ideal vaccination programme. The disease history in the region, the experience of the veterinarian and the available resources will play an important role in the definition of the strategy, and a few key points are important to consider regarding a Newcastle vaccination programme:
- The titres in the live vaccine
- The importance of the method of application
- The antigenic concentration in the inactivated vaccine
- The application of inactivated and live vaccines
If you are interested in more information about HIPRAVIAR® CLON and HIPRAVIAR® CLON/H120, both use the CL/79 strain, a cloned LaSota strain with lower post vaccine reaction compared to a normal LaSota strain, also both vaccines have a minimum titre of 10 6.5.
Highly concentrated inactivated vaccines to consider are the HIPRAVIAR® BPL2 and the HIPRAVIAR® ND BROILERS, inactivated LaSota strain Newcastle vaccines to provide long term protection.
You can access more detailed information about the vaccines by clicking on the links below:

| References | ||||
|---|---|---|---|---|
| Tonya L. Taylor, Patti J. Miller, Timothy L. Olivier, Enrique Montiel, Stivalis Cardenas Garcia, Kiril M. Dimitrov, Dawn Williams-Coplin, and Claudio L. Afonso | ||||
| (2017) | Repeated Challenge with Virulent Newcastle Disease Virus Does Not Decrease the Efficacy of Vaccines.. Avian Diseases | Vol. 61, No. 2, pp. 245-249. | ||
| Ingrid Cornax, Patti J. Miller, and, and Claudio L. Afonso | ||||
| (2012) | Characterization of Live LaSota Vaccine Strain–Induced Protection in Chickens upon Early Challenge with a Virulent Newcastle Disease Virus of Heterologous Genotype.. Avian Diseases Digest | September 2012, Vol. 7, No. 3, pp. e7-e8. | ||
| Cornax, I., Miller, P.J. and Afonso, C.L. | ||||
| (2012) | Characterization of Live LaSota Vaccine Strain–Induced Protection in Chickens upon Early Challenge with a Virulent Newcastle Disease Virus of Heterologous Genotype, genotypes III, VIg, VIb, VIId, and IX.. Avian Diseases | 56:464–470. | ||
| Alexander, D. | ||||
| (2003) | Newcastle Disease and Other Avian Paramixoviridae Infections. In: Diseases of Poultry, Cap 2. 11th Edition. Saif, Barnes, Glisson, Fadly, Mc Dougald, Swayne (eds.).. AAAP Iowa State University - USA. | 63-87. | ||
| Miller P.J and Koch G., | ||||
| (2013) | Newcastle Disease, Other Avian Paramyxoviruses, and Metapneumovirus Infections. Newcastle disease.. In: Diseases of Poultry, 13th Edition. Ed. By Swayne, D.E., Glisson, J.R., Mc Dougald, L.R., Nolan, L.K., and D.L. Suarez. Edit. Willey-Blackwell Publishing. Iowa, USA | p. 89-107. | ||
| Cornax, I., P. J. Miller, and C. L. Afonso. | ||||
| (2012) | Characterization of live LaSota vaccine strain-induced protection in chickens upon early challenge with a virulent Newcastle disease virus of heterologous genotype.. Avian Dis. | 56: 464-470. | ||
| Jeon, W. J., E. K. Lee, Y. J. Lee, O. M. Jeong, Y. J. Kim, J. H. Kwon, and K. S. Choi. | ||||
| (2008) | Protective efficacy of commercial inactivated Newcastle disease virus vaccines in chickens against a recent Korean epizootic strain.. J. Vet. Sci. | 9: 295-300. | ||
| J.L. Losada Torres, S.W. Ong, A. Rahman Omar, S.N. Azizah Mahmud. | ||||
| Evaluation of immunity with the application of Newcastle inactivated vaccine in different doses to day-old chicks.. CRWAD 2020. | ||||
| Miller, P. J., C. L. Afonso, A. J. El, K. M. Dorsey, S. C. Courtney, Z. Guo, and D. R. Kapczynski. | ||||
| (2013) | Effects of Newcastle disease virus vaccine antibodies on the shedding and transmission of challenge viruses.. Dev. Comp Immunol. | 41: 505-513. | ||
| Al-Garib, S. O., A. L. Gielkens, D. E. Gruys, L. Hartog, and G. Koch. | ||||
| (2003) | Immunoglobulin class distribution of systemic and mucosal antibody responses to Newcastle disease in chickens.. Avian Dis. | 47: 32-40. | ||
| Icochea, E.; Condemarin, A.; Losada, J.L.; Apaza, A.P.; Mora, B | ||||
| (2019.) | Comparative efficacy of different vaccination programs against Newcastle disease in commercial layer-type chickens introduction. WVPA, |









