



Nutritional Influences on Hatching Eggs
Professor Colin C. Whitehead, consultant at the Roslin Institute in Scotland, focuses on the importance of riboflavin, biotin, vitamin D and its metabolites as well as carotenoids in the broiler breeder diet, and how these can help the chicks to reach their potential in terms of performance and liveability.The importance of nutrition in optimising broiler breeder performance is well understood. However, the ultimate aim for broiler breeders is to produce chicks with maximum potential for subsequent performance and liveability.
Recent research has demonstrated the importance of the early nutritional status of the hatched chick in enhancing performance. It is therefore vital that, in formulating diets for the parent birds, attention is paid not only to maximising egg output and hatchability but also to ensuring that the eggs produced contain adequate amounts of the nutrients vital for supporting the early development of the hatching chick.
The micronutrient content of the egg is particularly influenced by the maternal diet and these nutrients must be supplied in amounts that fully meet the needs of the developing embryo and the hatching chick.
It is helpful to consider the mechanisms by which these nutrients enter the egg, for this gives insights into the requirement for the nutrient and also limits the inclusion of the nutrient.
Most vitamins are incorporated into eggs by specific transport mechanisms involving binding proteins. This is essential, because circulating levels in the maternal blood are not high enough to allow the amounts needed in the egg to accumulate by simple diffusion. An example of this is the incorporation of riboflavin (Rb) into eggs under the action of riboflavin binding protein (RBP).
RBP is a complex glycoprotein with a binding site for Rb and other phosphate rich sites that bind to receptors on the oocyte cell wall and enable RBP, whether or not it contains bound Rb, to be absorbed into the yolk.
Thus, riboflavin is actively incorporated into the developing yolk. It is also concentrated into albumen by a mechanism involving transfer from the plasma to a RBP synthesised in the oviduct.
The concentration of RBP in hen plasma is constant and the fractional saturation of RBP with Rb increases with the dietary supply of Rb. Maximal saturation of RBP is about 80 per cent in the yolk but is lower in the albumen (about 40 per cent), perhaps because of a limitation in the plasma to oviduct transfer of Rb.
However, once RBP is maximally saturated with Rb, further increases in the dietary supply are ineffective in increasing the egg content of Rb. Little, if any, free Rb is found in either yolk or albumen. These relationships are shown in Figure 1 and indicate why there is little increase in egg Rb contents with dietary concentrations above 10mg Rb/kg.
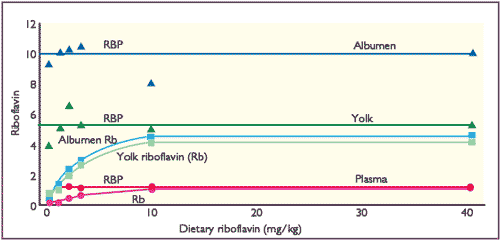
(White et al, 1986)
There are other binding proteins for other vitamins, though some of the principles may vary. Biotin is incorporated into yolk attached to two biotin binding proteins (BBP-I and BBP-II) and into albumen attached to a third, avidin.
However, biotin can induce the production of BBP-II so that the total concentration of BBP increases with dietary biotin content, until a limit is reached to the production of BBP.
Incorporation of biotin tends to plateau at this point, though some additional free biotin can appear in yolk suggesting that some diffusion of biotin from plasma occurs at higher dietary biotin concentrations.
Trace minerals are also incorporated into eggs by proteins, for example zinc is incorporated into yolk bound to vitellogenin.
The situation regarding the optimum incorporation of vitamin D and its metabolites into eggs is more complex. Vitamin D binding proteins can bind both cholecalciferol (vitamin D3) and 25-hydroxyvitamin D3 (25-D, available commercially as HyD) and transport these molecules into yolk.
The chick embryo can produce the liver 25-hydroxylase and renal 1- and 24-hydroxylases necessary for the production of the more active metabolites, including 1,25- dihydroxyvitamin D (1,25-D). In contrast, 1,25-D itself is poorly incorporated into eggs and feeding only this form of vitamin D to hens does not sustain hatchability.
Further injections of 1,25-D into eggs only partially restores hatchability and there is evidence that another di-hydroxylated metabolite, 24,25-dihydroxyvitamin D, is needed in addition to 1,25-D for full hatchability. Thus, both cholecalciferol and 25-D in the diet of the hen can meet the full needs of the hatching chick for vitamin D.
Data in Table 1 on the relationships between plasma 25-D concentrations in hens and hatching chicks suggest that the vitamin D status of the hatching chick, as assessed by plasma 25-D concentration, may be maximised by providing 25-D in the maternal diet.

HyD has been assessed widely in breeders as an extra feed supplement in addition to normal cholecalciferol. Small scale experiments under laboratory conditions may not always give statistically significant responses but industrial experience in a number of countries is suggesting that commercially important responses can be achieved in egg production, shell quality or hatchability.
This may suggest that the additional vitamin D status is beneficial under the more stressful conditions often experienced in large commercial flocks. The additional carryover to hatching chicks can also be helpful for their performance, not least in enhancement of early innate immune function.
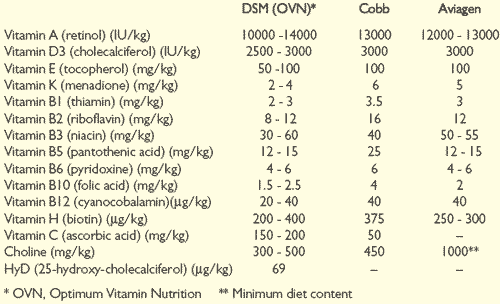
Suggested vitamin supplements for broiler breeder diets are shown in Table 2.
Carotenoids constitute a large group of lipid soluble compounds that are not traditionally thought of as nutrients. However, they have antioxidant and immunomodulatory functions and are important for pigmentation.
There is also a report that canthaxanthin supplementation (6mg Carophyll Red/kg) of a breeder diet based on white cereals improved hatchability.
Carotenoids can be incorporated into eggs from the diet of breeders in a dose-related manner and are carried over into the hatching chick. If hens have a low rate of egg production, carotenoids are accumulated in tissues and skin and, indeed, the presence of highly pigmented skin on a hen can be used to identify poor layers within a flock.
The incorporation of carotenoids into egg yolk can involve transport in different lipoprotein fractions and perhaps also specific binding proteins but appears to have a synergistic effect on incorporation of other fat soluble compounds into the egg and also in the young chick.
Thus Surai et al. (2003) have shown that addition of canthaxanthin (24mg/kg) to a breeder diet resulted in an increase in vitamin E uptake from the diet and transfer to the egg yolk. This was reflected in an increase in the liver vitamin E concentration of day-old chicks and a reduced rate of vitamin E depletion from liver over the first seven days post hatch.
Canthaxanthin has been shown to react with peroxyl radicals with adduct formation, so the decreased rate of vitamin E depletion may have been attributable to canthaxanthin having a sparing effect on vitamin E by directly scavenging free radicals.
Carotenoids can thus modulate antioxidant systems and decrease tissue susceptibility to oxidation. Other recent research has shown maternal effects of carotenoids on carotenoid uptake in growing chicks. Koutsos et al. (2003) compared carotenoid uptake in chicks hatching from eggs that were carotenoid replete or depleted through manipulation of the maternal diet. Chicks hatching from the replete eggs had higher tissue concentrations of carotenoids and showed much greater efficiencies of incorporation of carotenoids into immune tissues such as bursa and thymus and skin when fed diets containing lutein and canthaxanthin during the growing period.
Provision of carotenoids in the maternal diet may thus benefit the immune system of the young chick. The maternal use of carotenoids can also improve the cost effectiveness of subsequent carotenoid use for pigmentation purposes by increasing the efficiency of incorporation of carotenoids into skin in growing broilers.
This article was first published in International Hatchery Practice 2007, 21(4):9-10.
June 2010









