



Salmonella, mycoplasma and avian influenza monitoring in parent breeder flocks
The following breeder flock disease monitoring and testing protocols are based on the NPIP and are meant to serve as a guideline to breeder producers.The U.S. Department of Agriculture National Poultry Improvement Plan (NPIP) is a comprehensive federal, state, and industry cooperative program established to eradicate vertically transmitted diseases of poultry including Salmonella Pullorum and Gallinarum (typhoid). In later years, Mycoplasma gallisepticum and synoviae (MG and MS), Salmonella Enteriditis (SE), and low pathogenic avian influenza (LPAI) were also added to the program as a means to certify breeding flocks and encourage freedom of these pathogens. The NPIP also certifies sampling methodologies and specific diagnostics for all program diseases. The program is voluntary, but remains the gold standard for export certification and domestic sales of breeding stock and commercial pullets.
The NPIP program standards and testing protocols can be reviewed at www.poultryimprovement.org. Other countries and regional bodies have similar poultry health programs to certify breeding flocks as free of various diseases.
The following breeder flock disease monitoring and testing protocols are based on the NPIP and are meant to serve as a guideline only. Local disease challenges, laboratory availability, and/ or export or local market requirements may require alternative testing and certifications. Always follow local regulations regarding breeder flock monitoring and testing protocols.
Veterinary Inspection
All Hy-Line U.S.A. parent stock (PS) and PS source farms are inspected by a USDAaccredited veterinarian at least every 30 days when used for export purposes. Birds are examined for productivity, clinical signs of disease, and compliance with welfare rules. Farm managers report important production data daily, and alert the veterinarian if there is increased mortality, drops in egg production, decreased egg size, reduced feed or water intake, and/or signs of clinical disease or other abnormalities in the flock. Many important poultry diseases are monitored only by regular veterinary inspection of the flock.
Salmonella Enteriditis
Salmonella Enteriditis (SE) is an important human food pathogen that has been associated with
the consumption of eggs. Control of SE and other important Salmonella species in breeder flocks
is the first step to controlling these pathogens in commercial flocks. In other countries, additional
Salmonella species are under official regulatory monitoring and control.
A. Collection
1. Hatcheries
a. At each hatch, hand swab at least one hatch basket from all source breeder flocks (one swab).
b. The hatch basket floor, dead embryos, egg shell should be included in the swab
c. Swabs are placed in a tube containing appropriate support media (e.g. doublestrength skimmed milk).
d. Samples are transported to the laboratory for culture or PCR testing.
2. PS farms
a. Before chick placement, collect and test environmental swabs after cleaning and disinfection to verify that the house is Salmonella-negative.
b. Test chick box paper (meconium) and/or first week chick mortality using culture or PCR.
c. Every 4 weeks: Collect environmental swab samples from breeder farm.
- For floor or slat houses: use bootie swabs to sample the floor/slat area (4 swabs). Use hand swabs to sample egg belts (1 swab) and nest box pads (1 swab).
- For cage houses: Sample walkways using bootie swabs (4 swabs). Using hand swabs sample manure belts or manure pit (1 swab), egg belts (1 swab) and cage floors (1 swab).
d. Swabs are placed in a tube containing appropriate support media (e.g. doublestrength skimmed milk).
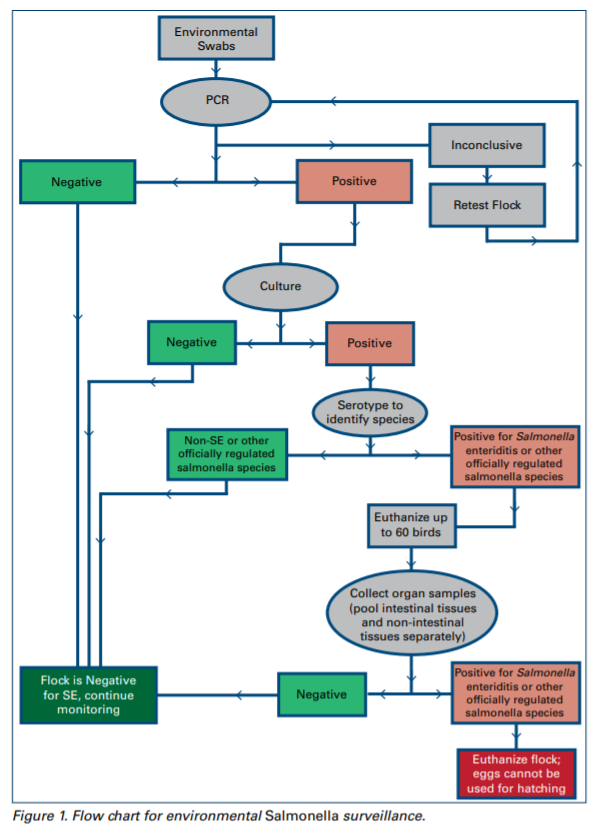
B. The status of the flock for salmonella should be determined by NPIP guidelines:
a. If PCR and/or culture results are negative, the flock is considered negative.
b. If PCR is inconclusive, the flock should be retested.
c. If PCR is positive, culture the environmental sample.
d. If culture of environmental sample is positive, colonies should be serotyped to identify salmonella species.
- Culture may be repeated for verification, and the flock treated as contaminated pending test results on the repeat culture.
e. Collect intestinal and other organ tissues for salmonella culture from 60 birds as described below.
- Intestinal tissues (collected separately from other organ tissues to avoid crosscontamination): digestive tract tissues including crop wall, duodenum, jejunum, ceca, cecal tonsil, and rectum/ cloaca.
- Other organs: liver, heart, pericardial sac, spleen, lung, kidney, peritoneum, gallbladder, oviduct, misshapen ova or testes, and inflamed or unabsorbed yolk sac. These organ samples are pooled together and processed as a single sample.
f. If the organ or intestinal cultures are positive for S. Enteriditis or other officially regulated salmonella species, the flock is considered positive.
Salmonella Pullorum and Salmonella Gallinarum
Salmonella Pullorum causes pullorum disease (bacillary white diarrhea) and S. Gallinarum (typhoid)
also causes major losses in young chicks. Both are vertically transmitted, and are difficult to culture
or detect from environmental samples. The most commonly used surveillance test for S. Pullorum
and S. Gallinarum (P-T) is rapid whole blood agglutination test to detect exposure.
1. At 16 weeks of age 300 birds (300 whole blood samples per flock) are tested using P-T whole blood agglutination test.
2. Retest the flock after the first qualifying test, 300 birds (300 whole blood samples per flock) using the P-T whole blood agglutination test.
3. In flocks that are vaccinated with inactivated bacterins or live Salmonella vaccines, leave 300 unvaccinated birds that are banded for identification for testing at 16 weeks of age, and again 12 months later.
4. The status of a flock for P-T should be determined according to NPIP rules:
a. If whole blood plate agglutination is negative, the flock is considered negative.
b. If whole blood plate agglutination is positive, euthanize 25 birds from the flock. Collect intestinal and other organ tissues from each bird for culture of S. Pullorumand S. Gallinarum as described below.
- Intestinal tissue pool (collected separately from other organ tissues to avoid cross-contamination): collect sample of crop wall, duodenum, jejunum, ceca, cecal tonsil, and rectum/ cloaca. These organ samples are pooled together and processed as a single sample, but do not pool samples from more than one bird.
- Other organs tissue pool (collected separately from intestinal tissues to avoid cross-contamination): collect organ tissues from liver, heart, pericardial sac, spleen, lung, kidney, peritoneum, gall bladder, oviduct, misshapen ova or testes, and inflamed or unabsorbed yolk sac. These organ samples are pooled together and processed as a single sample, but do not pool samples from more than one bird.
c. Swab any visibly abnormal tissue for culture.
d. If the organ or intestinal cultures are positive for S. Pullorum and S. Gallinarum, the flock is considered positive.
5. Breeder flocks confirmed as positive should be euthanized according to local regulations.
Mycoplasma Gallisepticum and Mycoplasma Synoviae
Mycoplasma gallisepticum and Mycoplasma synoviae can be passed vertically from breeder hen to
chick, resulting in poor chick quality, respiratory disease, synovitis, and reduced future production.
Breeder flocks participating in NPIP must remain MG and MS clean.
1. At 16 weeks of age, test 150 birds (150 serum samples per flock) using Serum Plate Agglutination Test or antibody Enzyme-linked Immunosorbent Assay (ELISA).
2. During the production period, test a total of 75 birds (75 serum samples) every 90 days or 25 birds (25 serum samples) every 30 days.
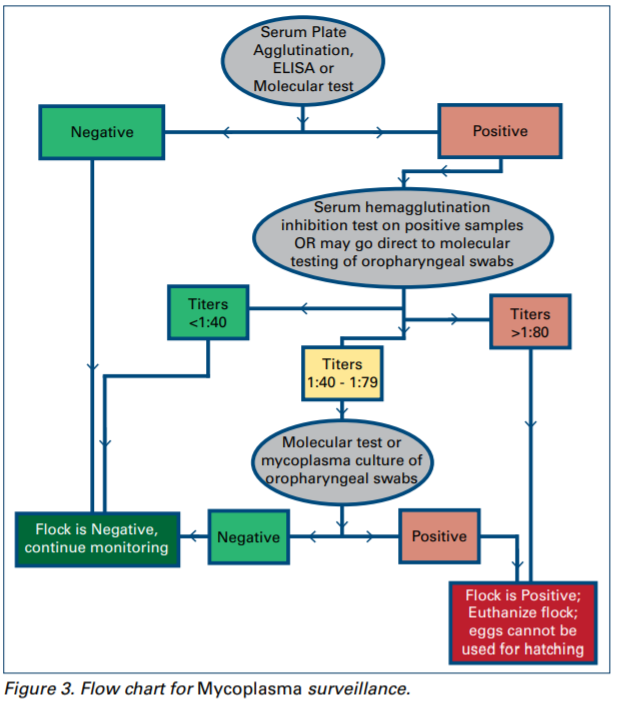
3. The status of the flock for MG and MS should be determined according to NPIP rules:
a. If the ELISA or serum plate test results are negative, the flock is considered to be negative.
b. If the ELISA or serum plate test results are positive, then perform hemaagglutination inhibition test on at least 25 samples or perform molecular test of oropharyngeal swabs. If hemagglutination test finds serum titers of 1:80 or greater, the flock is positive. Titers of 1:40 may be deemed suspicious and further testing ordered before a final judgement is made.
4. In high-risk areas, the testing interval can be shortened to every two weeks to detect exposure more rapidly.
5. Breeder flocks confirmed as positive should be euthanized according to local regulations.
Avian Influenza
Low pathogenic avian influenza surveillance was added to the NPIP to create more robust influenza monitoring and to include indemnity for producers that must euthanize a positive flock with H5 or H7 LPAI. By monitoring for low pathogenic H5/H7 LPAI infections it is easier to prevent reassortment events that may result in highly pathogenic avian influenza (HPAI) outbreaks. HPAI response remains under the authority of USDA-APHIS and requires controlled disposal of an affected flock.
1.At 16 weeks of age test 30 birds (30 serum samples) using antibody ELISA or Agar Gel Immunodiffusion (AGID).
2. After 16 weeks of age, test a total of 30 birds (30 serum samples) every 90 days or 10 birds (10 serum samples) every 30 days using AGID or ELISA.
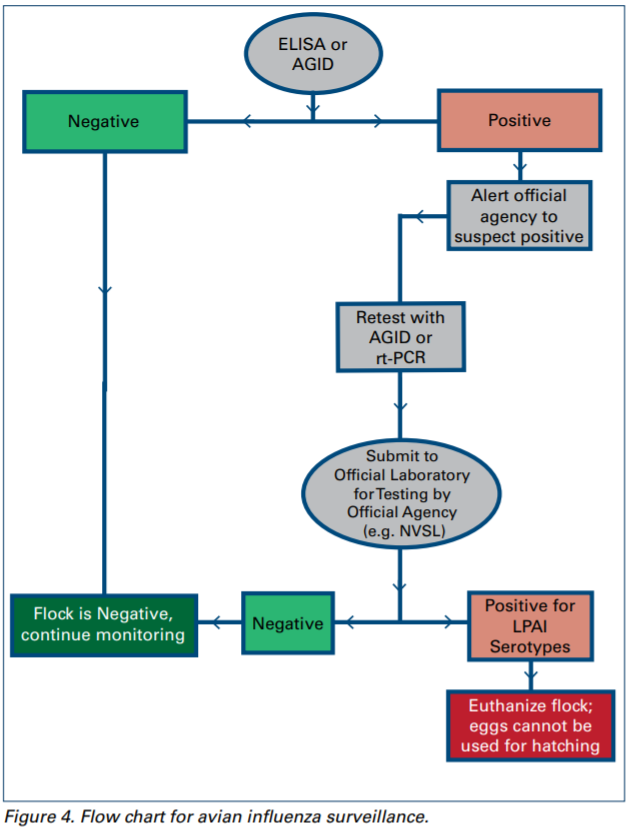
3. The status of a flock for avian influenza should be determined according to NPIP rules:
a. If ELISA or AGID is negative, the flock is considered negative.
b. If ELISA or AGID is positive, AGID or a molecular examination procedure (PCR) should be performed. Use oropharyngeal swabs, pooled 5 swabs per 3 ml (or 11 swabs per 5 ml) of appropriate transport media (BHI broth).
c. If AGID or PCR is positive, both serum samples and oropharyngeal/tracheal swabs should be sent out for verification and serotyping to the country of origin’s recognised national laboratory (in the U.S. ship to the National Veterinary Service Laboratory, NVSL).
4. In high-risk areas, reduce the testing interval to detect exposure more rapidly.
5. Flocks confirmed positive for LPAI may be euthanized according to local regulations
Surveillance Schedule
The following is a sample surveillance schedule for monitoring of breeder flocks for the diseases discussed.
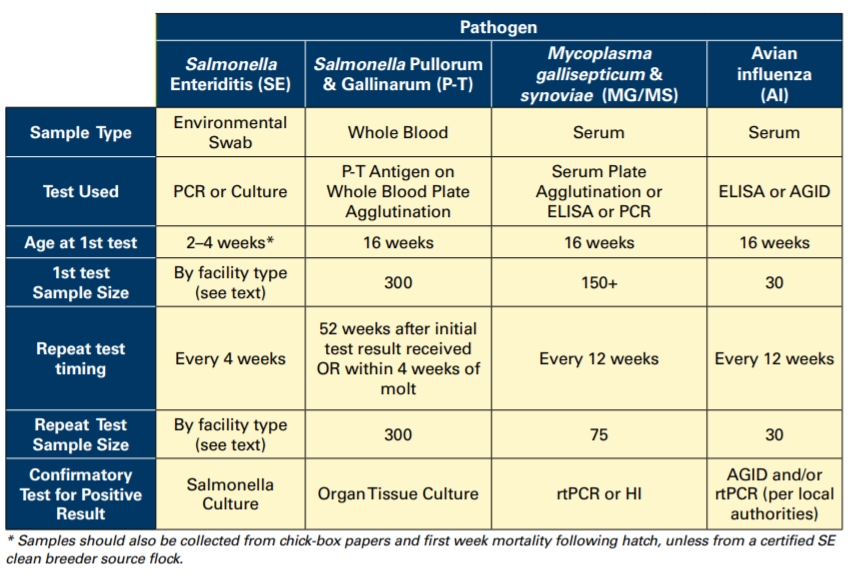
Best Practices for Surveillance
A more rigorous surveillance program requires a greater investment, but can enable faster identification of potential disease threats. Increased testing frequency, larger sample sizes, and using more sensitive screening tests can provide a faster response, and limit damage to business continuity, customer confidence, and public relations. Opting for a more intensive testing protocol may be particularly advantageous if a particular disease is of greater prevalence locally. Always follow local regulations regarding breeder flock monitoring and testing protocols as a minimum guideline.
For more information
National Poultry Improvement Plan (NPIP) www.poultryimprovement.org
Canadian Food Inspection Agency (CFIA) Hatchery Program http://www.inspection.gc.ca/animals/terrestrialanimals/hatchery/eng/1490636818761/1490636819401
British Egg Industry Council “Lion Code” http://www.britisheggindustrycouncil.co.uk/british-lion-code-of-practice/
Department for Environment, Farms & Rural Affairs (DEFRA) UK Poultry Health Scheme https://www.gov.uk/guidance/poultry-health-scheme-how-to-register
Technical Update, Proper Collection and Handling of Diagnostic Samples
- Part One: Serology and Blood Collection www.hyline.com/userdocs/pages/TU_SER1_ENG.pdf
- Part Three: Swabs www.hyline.com/userdocs/pages/TU_SER3_ENG.pdf
References
1. USDA-APHIS. “Part 145 - National Poultry Improvement Program or Breeding Poultry”. ElectronicCode of Federal Regulations. U.S. Government Publishing Office. 2017. https://www.ecfr.gov/ cgi-bin/text-idx SID=f7f70f0e0573ba9852400ac93b908a7d&mc=true&node=pt9.1.147&rgn=div5
2. USDA-APHIS. “Part 147 – Auxiliary Provisions on National Poultry Improvement Plan”. Electronic Code of Federal Regulations. U.S. Government Publishing Office. 2017. https://www.ecfr.gov/ cgi-bin/text-idx?SID=f7f70f0e0573ba9852400ac93b908a7d&mc=true&node=pt9.1.147&rgn=div5
3. USDA-APHIS. National Poultry Improvement Program Standards. U.S. Department of Agriculture.
2017.









