



The importance of nutrition on egg shell quality in broiler breeders
Poor egg shell quality and contaminated eggs are often the main factors to poor hatchabilityThe Egg Shell: What do we know?
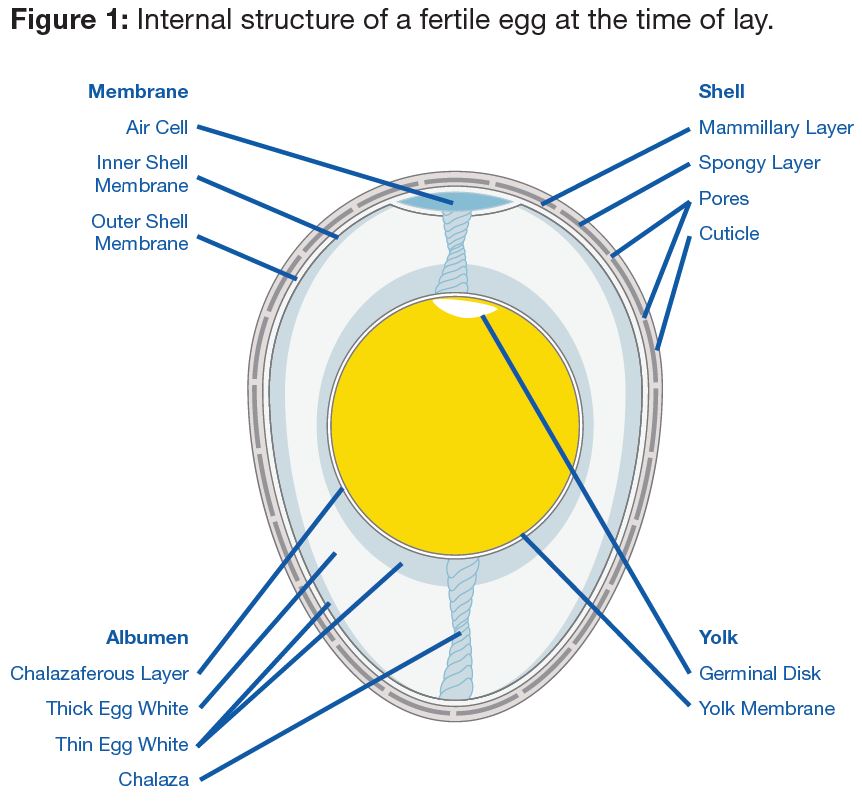
The egg shell protects and supports the internal soft structures. It is semi-permeable to air and water and helps prevent bacterial infection. About 94-95% of the dry egg shell is calcium carbonate (CaCO3) and weighs 5.5-6.0 g (0.19-0.21 oz) (Mongin, 1978). Good quality egg shells from broiler breeders comprise about 2.0-2.2 g (0.07-0.08 oz) of calcium in the form of CaCO3 crystals. A typical egg shell contains about 0.3% phosphorus and 0.3% magnesium and traces of sodium, potassium, zinc, manganese, iron and copper. The rest of the dry egg shell is made up of an organic matrix material which has calcium binding properties and its organization during shell formation plays a vital role in the strength of the egg shell. The strength of the shell is further dependent on the amount of shell present, relative to the egg size, shape and thickness.
Cuticle
The outermost part of the egg shell is the cuticle (Figure 1). The cuticle is a non-calcified, thin, water-insoluble coating composed mainly of glycoproteins. It renders the shell impervious to water and seals the pores on the shell to keep out dust and bacteria, but plays a role in regulating moisture and gaseous exchange during incubation and preventing desiccation (drying out) of the embryo.
© Aviagen
When the egg is laid, the cuticle is not completely stabilized; it appears wet for 2-3 minutes under a microscope, and will have an open, spongy appearance. After it matures, it hardens to a smoother surface. Until the cuticle is set, it will not protect the pores from bacterial penetration. If the egg is laid onto a dirty surface, then bacteria will almost certainly enter the egg shell and cause contamination in the internal egg contents and negatively impact embryo development.
Cracked Eggs
It is obvious that when an external force exceeds shell strength, egg breakage results. Cracked eggs can be either complete (when both the shell and shell membranes are broken) or incomplete (when the shell is broken, but the shell membranes stay intact). Completely cracked eggs are not used for hatching due to the high risk of severe moisture loss and bacterial contamination. However, incomplete hairline-cracked eggs are less obvious upon visual inspection and may inadvertently be used in commercial hatcheries.
There are also external egg quality issues related to other shell defects which do not necessarily result in egg breakage. These include rough shells, misshapen eggs, body-checked eggs, shell-less eggs and stained or dirty floor eggs. These occur less frequently than those associated with actual shell strength problems, but nevertheless can give rise to increased risks of contamination or lower hatchability.
Issues with Poor Egg Shell Quality
Barnett et.al., (2004) conducted research to determine whether chicks from eggs with hairline cracks would hatch and grow normally compared to those with undamaged shells. They found that eggs with hairline cracks resulted in significantly poorer hatch of fertile, greater egg weight loss and higher embryonic mortality.
In another study using specific gravity as a determinant of egg shell thickness, Roque and Soares (1994), discovered that thick-shelled eggs (specific gravity 1.080) showed increased hatchability and lower intermediate and late embryonic mortality.
What Influences Egg Shell Quality?
A range of both nutritional and non-nutritional factors could influence the quality of an egg shell in broiler breeders. These include:
- Time duration the egg spends in the shell gland during shell formation
- Rate of calcium deposition in the shell gland
- Time of day the egg is laid
- Age of hen; thickness declines with age and egg size increases
- Infectious agents/diseases and contamination (e.g. Infectious Bronchitis, Egg Drop Syndrome, Newcastle Disease, Mycoplasma, T-2 and HT-2 mycotoxins; sulphonamides, organochloride insecticides)
- Nutritional deficiencies and excesses
- Saline drinking water
- Time of feeding
- Others – such as genotype, housing or production system, environment (temperature, lighting, water availability and quality), general stress, management practices (including flock uniformity and egg handling)
The Importance of Optimal Nutrition
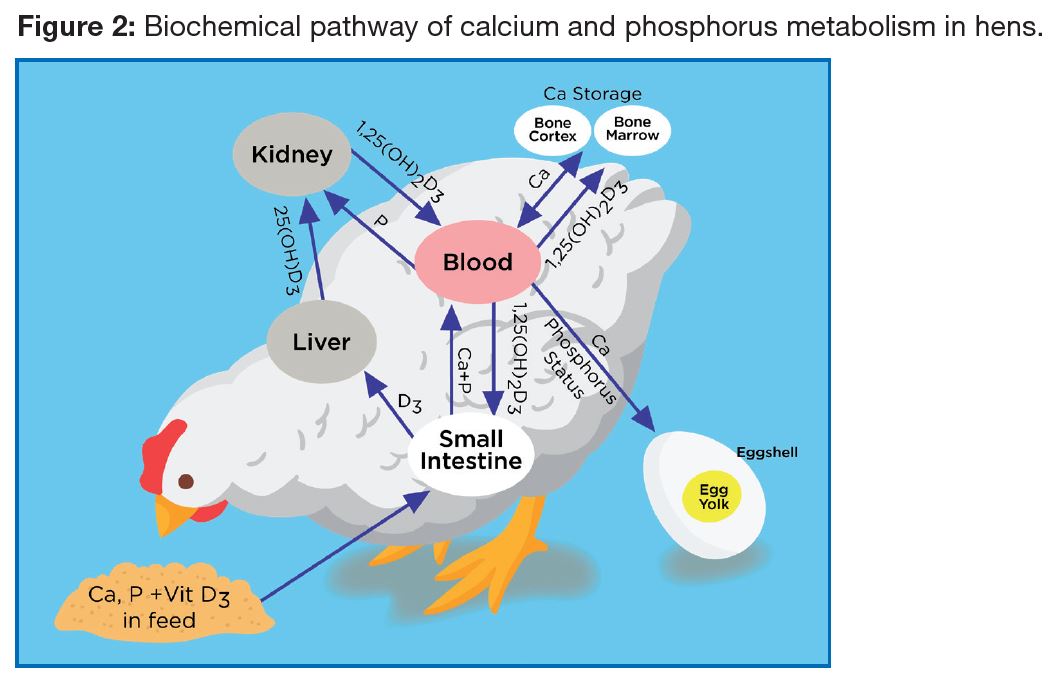
As the egg shell mostly contains CaCO3, it is normally assumed that calcium is the only nutrient responsible for shell quality. However, both phosphorus and Vitamin D3 are also involved (Figure 2), together with a number of trace minerals. Gut health status and kidney function play important roles in calcium absorption and Vitamin D3 activity.
© Aviagen
Calcium is needed by broiler breeder hens at adequate levels (up to 4.9-5.1 g/bird/day; 0.17-0.18 oz/bird/day) for egg shell formation. An adequate source and supply of calcium during lay is crucial to prevent the occurrence of several issues, including:
- calcium tetany,
- skeleton abnormalities and
- poor egg shell quality (thin, soft shelled, cracked eggs).
In addition, ovulation disruption, un-sustained early egg production, egg production drops and halt (especially in larger bodied, early maturing pullets) can also occur. In a controlled-fed broiler breeder the quantity of calcium consumed varies according to actual feeding allocation. The particle size and origin of calcium source (e.g. oyster shell, limestone) must be taken in to account when establishing the dietary calcium level during lay. An adequate calcium balance is important in order to avoid deficiencies or excesses with negative impacts on egg shell quality due to either insufficient calcium mineralization or utilization of important minerals such as phosphorus, magnesium, manganese and zinc. Due to high dietary calcium content of layer diets, and consequential impact on dietary bulk density, there is a tendency for feed particle size segregation and can lead to more variation in dietary calcium analysis results. Thus, it is also important to ensure proper feed mixing times to achieve a high level of accuracy between formulated and actual finished feed dietary levels.
Phosphorus is present at a low level in the egg shell, but is important for replenishing the hen’s medullary bone. There must be sufficient dietary phosphorus to assimilate calcium into the bone matrix. Therefore, providing sufficient daily intake of “available” phosphorus (500-585 mg/bird/day or 0.018-0.020 oz/bird/day from peak to depletion)
is critical for optimal egg shell quality. Requirements can change under heat stress conditions and it is important to avoid hypophosphatemia (Hopkinson et.al., 1984). Conversely, a high level of available phosphorus (in terms of
non-phytate phosphorus or NPP) can have negative effects. Researchers Ekmay and Coon (2011), have shown that reducing NPP improves specific gravity of eggs. They also found that even at the lowest intake of NPP (0.2%) egg production was sustained. Based on this research, an argument can be made that dietary available phosphorus for broiler breeders should be limited to ≤ 0.35%, particularly after 35 weeks of age.
Vitamin D3 is an important vitamin involved in calcium metabolism in both liver and kidney and hence significantly affects egg shell quality. Vitamin D3 is required for normal calcium absorption. An inadequate amount of dietary Vitamin D3 quickly induces calcium deficiency and a drop in egg shell weight, resulting in weaker and thinner shells. A minimum dosage of 3,500 IU/kg (1587 IU/lb) of Vitamin D3 in broiler breeder feeds is recommended for egg production, shell quality and hatchability. Under field challenge circumstances involving liver or kidney integrity, some commercial Vitamin D metabolites have proven to promote increased calcium retention in the bird and improve shell quality.
Trace minerals such as manganese, copper and zinc are important to achieve good egg shell quality. The levels recommended by primary breeding companies for these nutrients should satisfy requirements for shell quality. It is important to adopt reliable and well defined mineral sources. There might be benefits in terms of bio-availability in supplying part of these minerals from organic sources in enhancing egg shell quality (Stefanello et.al., 2014).
Electrolytes are involved in acid-base balance (Na+K-Cl); this is also referred to as electrolyte balance and is one of the major metabolic factors involved in the egg shell formation (Mongin, 1978). Under normal conditions ensuring an electrolyte balance around 200 mEq/kg (90.7 mEq/lb) of feed is sufficient to ensure optimal egg shell quality. Heat stressed birds often lay eggs with thinner, weaker shells because of blood acid-base balance disturbances as a result of panting (hyperventilation). Hyperventilation leads to excessive loss of CO2 gas from the blood. Lower CO2 causes blood pH to elevate or become more alkaline. The higher blood pH reduces the amount of ionized Ca and CO3 delivered to the uterus for egg shell formation. Increasing the amount of calcium in the feed does not correct this problem. However, under practical conditions replacement of part (30-35%) of the salt (NaCl) with sodium bicarbonate (NaHCO3) and increasing the K level to achieve an electrolyte balance above 200 mEq/kg has proven to be beneficial for egg shell strength. Evidence suggests that addition of Vitamins C and E (200 mg/kg and 250 mg/kg in a breeder diet, respectively) can improve egg specific gravity and shell thickness substantially in broiler breeders under prolonged heat stress (Chung et.al., 2005).
Saline drinking water, which is high in sodium and chloride can inhibit the activity of carbonic anhydrase enzyme in the shell gland mucosa which limits supply of bicarbonate ions (and calcium) in the lumen of the shell gland to form CaCO3 (Chen and Balnave, 2001). Not many controlled studies have been conducted in broiler breeders in this area in contrast with commercial layers. Older broiler breeder hens (> 40 weeks) are known to be more sensitive to saline water and have a reduced capacity to recover from the adverse effects of high NaCl. Reducing NaCl in feed has little offset potential and so the best remedy for high levels of NaCl in drinking water is desalination (reverse osmosis), and to avoid drinking water with ≥ 500 ppm NaCl.
Time of feeding can influence shell quality. Broiler breeders are usually fed during the early morning hours. Unfortunately, this does not coincide with the time of egg shell deposition (calcification). Peak demand for calcium occurs during the night when egg shell deposition occurs. Since there is a limited amount of calcium in the digestive tract at the time of egg shell calcification, a significant amount of calcium is mobilized from the skeletal system for shell formation. Research findings indicate that the more skeletal calcium is used in shell formation, the poorer is shell quality (Leeson and Summers, 2000).
Farmer et.al., (1983) found that better egg shell quality was achieved when broiler breeders were fed late in the afternoon compared to birds fed in the early morning. This was due to the fact that significantly more calcium was available in the digestive system during shell calcification. In practice, feeding in the late afternoon or evening may not be feasible but it is worthy of consideration if shell quality on a farm is poor. This is especially applicable for older flocks as both efficiency of calcium absorption from the gut and skeletal resorption decline with age.
Calcium source particle size is an alternative option to evening feeding via supplemental use of large limestone grits (2-4 mm size) or oyster shell. Coarse calcium grits or particles are retained longer in the gizzard, reduce calcium solubility and help extend calcium absorption from feed into the night time period. Application at the farm during late afternoon in the feed trough or on the litter can enhance overall shell quality in aged hens by increasing shell weight per unit surface area and egg shell content.
Many studies have shown the advantages of coarse calcium sources in enhancing shell quality, particularly with older breeders. An investigation was undertaken in a commercial broiler breeder flock by Reis et.al., (1995) to examine the effects of coarse supplemental limestone on egg shell quality and subsequent incubation outcome. Compared to broiler breeders given a regular breeder diet containing 3.1% calcium at 8:00 a.m., birds on the same feeding program but supplemented with 2 g/bird/day (0.07 oz/bird/day) of coarse limestone in the afternoon had markedly better egg specific gravity, but egg weight loss during incubation was not altered. Hatchability and chick viability were significantly improved with supplemental limestone feeding. Most of the improvement in hatchability and chick viability was the result of a lowered incidence of egg contamination. It is probable that thicker shelled eggs are less susceptible to bacterial penetration.
Egg Size Management
Hens lay heavier eggs as they age and increase in body weight; however, the egg shell becomes increasingly thinner, as there is no proportionate increase in shell weight. At the same time, the ability of the hens to absorb calcium in the intestines reduces. Hence farms with ageing flocks may encounter a higher incidence of shell problems and a drop in hatchability. One way of controlling shell problems in older broiler breeders is to manage egg size. This can be achieved by adopting a 3-stage feeding program with decreasing protein and amino acids as the birds age (Table 1). This will help control body weight, achieve target egg weights, support persistency of lay, and improve fertility and hatchability.
Table 1: The Aviagen® 3-phase broiler breeder PS nutrient recommendations for Ross® broiler breeders.
Nutrient in diet | Breeder 1 (5%-35 wk) | Breeder 2 (35-50 wk) | Breeder 3 (>50 wk) |
ME (kcal/kg) | 2800 | 2800 | 2800 |
Crude protein (%) | 15.0 | 14.0 | 13.0 |
Dig Lysine (%) | 0.60 | 0.56 | 0.52 |
Dig. Meth and Cyst (%) | 0.59 | 0.57 | 0.54 |
Calcium (%) | 3.00 | 3.20 | 3.40 |
Av Phosphorus (%) | 0.35 | 0.33 | 0.32 |
Sodium (%) | 0.18-0.23 | 0.18-0.23 | 0.18-0.23 |
Chloride (%) | 0.18-0.23 | 0.18-0.23 | 0.18-0.23 |
Potassium (%) | 0.60-0.90 | 0.60-0.90 | 0.60-0.90 |
Manganese (mg/kg) | 120 | 120 | 120 |
Zinc (mg/kg) | 110 | 110 | 110 |
Copper (mg/kg) | 10 | 10 | 10 |
Vitamin D3 (IU/kg) | 3,500 | 3,500 | 3,500 |
Summary
Thin egg shells and contaminated eggs greatly affect hatchability in broiler breeders. Good farm biosecurity and management practices are imperative in preventing diseases and providing a favorable bird environment. Adopting a proper egg handling process and having an effective quality control program in the hatchery are important to ensure good hatchability.
Appropriate nutrition and feeding programs are critical to control body weight and egg size to breeder performance objectives and to achieve satisfactory shell quality. Breeder diets need to be formulated to nutrient levels advised by the primary breeder to provide optimal levels of calcium, phosphorus, Vitamin D3 and important trace minerals. Use of a combination of D3 and 25-hydroxy-D3 metabolite and organic trace minerals are considered worthwhile.
If poor shell quality is a recurring issue in breeder flocks - check birds’ drinking water for salinity (NaCl), apply supplemental coarse limestone where practical and consider late afternoon feeding. In prolonged heat stress conditions, in conjunction with extra Vitamin E and C, it is advisable to replace part of the dietary NaCl with sodium bicarbonate (NaHCO3) to attain a proper dietary electrolyte balance.
References
Barnett D.M., B.L. Kumpula, R.L. Petryk, N.A. Robinson, R.A. Renema, and F.E. Robinson. 2004. Hatchability and early chick growth potential of broiler breeder eggs with hairline cracks. J. Appl. Poult. Res. 13:65-70.
Chen J., and D. Balnave. 2001. The influence of drinking water containing sodium chloride on performance and egg shell quality of a modern, colored layer strain. Poult. Sci. 80:91-94.
Chung M.K., J.H. Choi, Y.K. Chung, and M. Chee. 2005. Effects of dietary vitamins C and E on egg shell quality of broiler breeder hens exposed to heat stress. Asian-Aust. J. Anim. Sci.18:545-551.
Ekmay R.D. and C.N. Coon. 2011. An examination of the P requirements of broiler breeders for performance, progeny quality and P balance. 2. Ca particle size. Int. J. Poult. Sci. 10:760-765.
Farmer M., D.A. Roland, and M.K. Eckman. 1983. Calcium metabolism in broiler breeder hens. 2. The influence of the time of feeding on calcium status of the digestive system and egg shell quality in broiler breeders. Poult. Sci. 62:465- 471.
Hopkinson, W.I., W. Williams, G.L., Griffiths, D. Jessop, and S.M, Peters. 1984. Dietary Induction of sudden death syndrome in broiler breeders. Avian Dis. 28:352-357.
Leeson S., and J.D. Summers. 2000. Broiler Breeder Production. Nottingham University Press, Thrumpton, Nottingham, England (2000), pp. 136-217.
Mongin, P., 1978. Acid-base balance during eggshell formation in Respiratory Function in Birds. Adult and Embryonic.
J. Piiper, ed. Springer-Verlag, New York, NY. pp. 247-259.
Reis L.H., P. Feio, L.T. Gama, and M.C. Soares. 1995. Extra dietary calcium supplement and broiler breeders. J. Appl. Poultry Res. 4:276-282.
Roque L. and M.C. Soares. 1994. Effects of egg shell quality and broiler breeder age on hatchability. Poult. Sci. 73:1838-1845.
Stefanello, C., T.C., Santos, A.E., Murakami, E.N. Martins, and T.C. Carneiro. 2014. Productive performance, egg shell quality, and egg shell ultrastructure of laying hens fed diets supplemented with organic trace minerals. Poult. Sci. 93:104-113.









