The use of vaccination as an option for the control of Avian Influenza
By Ilaria Capua and Stefano Marangon for the Office International des Epizooties - Recent epizootics of highly contagious OIE List A diseases, such as foot and mouth disease, classical swine fever and avian influenza (AI), led to the implementation of stamping-out policies resulting in the depopulation of millions of animals.
Summary
The enforcement of a control strategy that is based only on the application of sanitary restrictions on farms and that involves the culling of animals that are infected, suspected of being infected or suspected of being contaminated, may not be sufficient to avoid the spread of infection, particularly in areas that have high animal densities, and thus results in mass depopulation.
In the European Union, the directive that imposes the enforcement of a stamping-out policy (92/40/EC) for AI was adopted in 1992, although it was drafted in the 1980s. The poultry industry has undergone substantial changes in the past 20 years, mainly resulting in shorter production cycles and in greater animal densities per territorial unit. Due to these changes, infectious animal diseases are significantly more difficult to control because of the greater number of susceptible animals reared per given unit of time and to the difficulties in applying adequate biosecurity measures.
The slaughter and destruction of large numbers of animals is also questionable from an ethical point of view, particularly when the human health implications are negligible. Mass depopulation has raised serious ethical concerns among the general public, and has recently given rise to high costs and economical losses for governments, stakeholders and, ultimately, for consumers.
In the past, the use of vaccines in such emergencies was limited by the inability to differentiate vaccinated/infected from vaccinated/non-infected animals. The major concern was that the disease could spread further through trade or movement of apparently uninfected animals or products of animal origin, or that the disease might be exported to other countries. For this reason export bans have been imposed on countries enforcing a vaccination policy.
This paper reviews possible strategies for the control of AI infections bearing in mind the new definition of AI proposed by the OIE. An overview of the advantages and disadvantages of using conventional inactivated (homologous and heterologous) vaccines and recombinant vaccines is presented and discussed in detail. Reference is made to the different control strategies, including the movement restriction measures to be applied when a vaccination policy is enforced. The implications for trade of a vaccination policy are discussed.
In conclusion, if vaccination is accepted as an option for the control of AI, vaccine banks, including companion diagnostic tests, must be established and made available for immediate use.
1. Introduction
Recent epizootics of highly contagious OIE List A diseases, such as foot and mouth disease, classical swine fever and avian influenza (AI), have led to the implementation of stamping-out policies resulting in the depopulation of millions of animals. The implementation of a control strategy that is based only on the application of sanitary restrictions and that involves the culling of animals that are infected, suspected of being infected or suspected of being contaminated, may not be sufficient to avoid the spread of infection. This is particularly so in areas with high animal densities where such a control strategy results inevitably in mass depopulation. There is an increased risk of disease spread in these areas, and the financial consequences of the occurrence of an epizootic are severe (4, 11, 14, 17).
With regard to AI, the European Union (EU) directive that imposes the enforcement of a stamping-out policy (92/40/EC) was adopted in 1992, although it was drafted in the 1980s (7). The poultry industry has undergone substantial changes in the past 20 years, mainly resulting in shorter production cycles and in greater animal densities per territorial unit. Due to these organisational changes, infectious diseases are significantly more difficult to control because of the greater number of susceptible animals reared per given unit of time and to the difficulties in applying adequate biosecurity programmes. In order to avoid the destruction of large numbers of animals, the possibility of pursuing different control strategies should be considered.
The slaughter and destruction of large numbers of animals is also questionable from an ethical point of view, particularly when the implications for human health are negligible. Mass depopulation has raised serious ethical concerns among the general public. The policy has also led to very high costs and economical losses for the EU community budget, the EU Member States, the stakeholders and, ultimately, for the consumers.
In the EU, the use of vaccines in such emergencies has been limited by the inability to differentiate vaccinated/infected from vaccinated/non-infected animals. The major concern was that the disease could spread further through trade or movement of vaccinated animals or their products, or that the disease might be exported to other countries, primarily because it was not possible to establish whether the vaccinated animals had been field exposed to the disease agent.
This paper reviews the possible strategies for the control of AI infections, bearing in mind the new definition of AI proposed by the EU (Document Sanco/B3/AH/R17/2000; ref.12) and by the OIE (Ad hoc Group on Avian Influenza, OIE International Animal Health Code Commission meeting of 29–30 October 2002) and the possibility of enforcing an emergency vaccination programme with the vaccines available currently. Reference will be made to the type of vaccines available, the efficacy of these vaccines, their limitations, and the possibility of identifying infected animals in a vaccinated population.
Definition of avian influenza
AI viruses all belong to the influenza virus A genus of the Orthomyxoviridae family and are negative-stranded, segmented RNA viruses. The influenza A viruses, can be divided into 15 subtypes on the basis of the haemagglutinin (H) antigens. In addition to the H antigen, influenza viruses possess one of nine neuraminidase (N) antigens. Virtually all H and N combinations have been isolated from birds, thus indicating the extreme antigenic variability that is a hallmark of these viruses. Changes in the H and N composition of a virus may be brought about by genetic reassortment in host cells. One of the consequences of genomic segmentation is that if co-infection by different viruses occurs in the same cell, progeny viruses may originate from the reassortment of parental genes derived from different viruses. Thus, as the influenza A virus genome consists of eight segments, 256 different combinations of progeny viruses may arise theoretically from two parental viruses.
Current EU legislation (7) defines AI as ‘an infection of poultry caused by any influenza A virus that has an intravenous pathogenicity index in 6-week-old chickens greater than 1.2 or any infection with influenza A viruses of H5 or H7 subtype for which nucleotide sequencing has demonstrated the presence of multiple basic amino acids at the cleavage site of the haemagglutinin’. However it has been proved that highly pathogenic avian influenza (HPAI) viruses emerge in domestic poultry from low pathogenicity (LPAI) progenitors of the H5 and H7 subtypes. It therefore seems logical that HPAI viruses and also their LPAI progenitors must be controlled when they are introduced in domestic poultry populations (12). The new proposed definition of AI by the OIE and the EU (12) is ‘an infection of poultry caused by either any influenza A virus that has an IVPI (intravenous pathogenicity index) in 6-week-old chickens greater than 1.2 or any influenza A virus of H5 or H7 subtype’. With reference to the present paper, the term AI applies to all AI viruses of the H5 and H7 subtype, regardless of their virulence and of their pathogenicity for domestic poultry.
2. Rationale behind the use of vaccines
When an outbreak of AI occurs in an area with a high population density in which the application of rigorous biosecurity measures is incompatible with the modern rearing systems, vaccination should be considered as a first option to control the spread of infection. The expected results of the implementation of a vaccination policy on the dynamics of infection are primarily those of reducing susceptibility to infection (i.e. a higher dose of virus is necessary for establishing productive infection) and reducing the amount of virus shed into the environment. This association between a higher infective dose needed to establish infection and less virus contaminating the environment represents a valuable aid to the eradication of infection.
Clearly, the efficacy of an emergency vaccination programme is inversely correlated to the time span between the diagnosis in the index case and the implementation of mass vaccination. For this reason, it is imperative that if emergency vaccination is to be considered as a possible option in a given country, vaccine banks must be available in the framework of national contingency plans.
3. Currently avialable vaccines
Conventional vaccines
Inactivated homologous vaccines: These vaccines were originally prepared as ‘autogenous’ vaccines, i.e. vaccines that contain the same AI strain as the one causing problems in the field. They have been used extensively in Mexico and Pakistan during the AI epizootics (22).
The efficacy of these vaccines in preventing clinical disease and in reducing the amount of virus shed in the environment has been proven through field studies and experimental trials (22). The disadvantage of this system is the impossibility of differentiating vaccinated from field-exposed birds unless unvaccinated sentinels are kept in the shed. However, the management (identification, bleeding and swabbing) of sentinel birds during a vaccination campaign is time-consuming and rather complicated, as they are difficult to identify and they may be substituted with seronegative birds in the attempt to escape the restrictions imposed by public health officials.
Inactivated heterologous vaccines: These vaccines are manufactured in a similar way to inactivated homologous ones. They differ in that the virus strain used in the vaccine is of the same H type as the field virus but has a heterologous neuraminidase. Following field exposure, clinical protection and reduction in viral shedding are ensured by the immune reaction induced by the homologous H group, while antibodies against the neuraminidase induced by the field virus can be used as a marker of field infection (5).
For both homologous and heterologous vaccines, the degree of clinical protection and the reduction in viral shedding are improved by a higher antigen mass in the vaccine (18). For heterologous vaccines the degree of protection is not strictly correlated to the degree of homology between the haemagglutinin genes of the vaccine and challenge strains (22). This is definitely a great advantage as it enables the establishment of vaccine banks because the master seed does not contain the virus that is present in the field and may contain an isolate (preferably of the same lineage) available before the epizootic.
Recombinant vaccines
Several recombinant fowlpox viruses expressing the H5 antigen have been developed (1, 2, 20, 21, 24) and one has been licensed and is being used currently in Mexico (22). Experimental data have also been obtained for fowlpox virus recombinants expressing the H7 antigen (3). Other vectors have been used to successfully deliver the H5 or H7 antigens, such as constructs using infectious laryngotracheitis virus (ILTV) (16).
The only field experience with a recombinant virus to control AI has been obtained in Mexico (23), where it has been used in the vaccination campaign against a LPAI H5N2 virus.
No such product has been licensed in the EU to date.
Until recent times, vaccination against AI viruses of the H5 and H7 subtypes was not considered or practised in developed countries because of export bans on live poultry and on poultry products (8). Export bans have also been imposed in cases of infection with a H5 or H7 virus, regardless of the virulence of the isolate. Export bans frequently represent the major cause of economic loss due to the occurrence of an OIE List A disease.
While the severe clinical signs caused by HPAI ensure a prompt diagnosis and facilitate the implementation of a stamping-out policy, the inconspicuous nature of the disease caused by viruses of low pathogenicity make this infection difficult to diagnose. Detection of infection is only possible by the implementation of appropriate surveillance programmes. Bearing in mind the new proposed definition of AI, and the potential mutation of LPAI of the H5and H7 subtypes to HPAI, it is easy to understand why these bans have been imposed. For the sake of trade, freedom from AI should be demonstrated in a given country or zone by ongoing surveillance programmes. This approach is supported by the fact that in several recent outbreaks, infection with a virus of low pathogenicity was only detected once infection was widespread, and often out of control.
In the absence of vaccination, trade bans imposed on a given area last until freedom from infection can be demonstrated in the affected population. Prolonged trade bans are also imposed when a vaccination policy is adopted that does not enable the application of a ‘DIVA’ (Differentiating Infected from Vaccinated Animals) strategy (either for the type of vaccine used or because the monitoring system in place does not guarantee that infection is no longer circulating). On the contrary, if it is possible to demonstrate that the infection is not circulating in the vaccinated population, trade bans may be lifted.
Such ‘marker’ vaccination strategies offer attractive control options for OIE List A diseases. In case of an outbreak of AI in a densely populated poultry area (DPPA) the option of vaccinating should be pursued. To safeguard international trade, a control strategy that enables the differentiation between vaccinated/infected and vaccinated/ non-infected animals should be implemented. The possibility of using vaccines would support restriction-based control measures, thus reducing the risk of a major epizootic and the subsequent mass stamping-out policy.
5. Options for control
It is extremely difficult to establish fixed rules for the control of infectious diseases in animal populations because due to the unpredictable number of variables involved. With regard to AI however, some basic scenarios may be hypothesised; some guidelines for the application of control policies based on the considerations made above are shown in Table 1.
| Table 1. Guidelines for the application of control policies for AI | ||||
| H5/H7 virus pathogenicity | Index case flock | Evidence of spread to industrial sector | Population density in area | Policy |
| HPAI/LPAI | Backyard | No | High/Low | Stamping-out |
| HPAI/LPAI | Backyard | Yes | Low | Stamping-out |
| High | Vaccination | |||
| HPAI/LPAI | Industrial | No | High/Low | Stamping-out |
| HPAI/LPAI | Industrial | Yes | Low | Stamping-out |
| High | Vaccination | |||
There are several crucial steps that must be carried out if AI represents a risk. First, the index case must be identified promptly. This does not represent a problem if the virus is of high pathogenicity, but it can be a serious concern if the virus if of low pathogenicity. For this reason countries or areas at risk of infection should implement specific surveillance systems to detect infection with LPAI as soon as it appears.
Secondly, a timely assessment of whether there has been spread to the industrial poultry population in the area must be performed. This is a crucial evaluation that must be made available to decision makers.
Once an AI outbreak has been identified, eradication measures based on stamping out or controlled marketing of slaughterbirds on infected farms must be enforced. The choice between these two options must be taken bearing in mind the pathogenicity and transmissibility of the virus, the density of poultry farms around the affected premises, the economic value of the affected birds, and the logistics of carrying out a slaughter/stamping-out policy. In Italy, a stamping-out policy was generally applied to LPAI-infected young meat-birds, breeders and layers, while controlled marketing was applied to older meat-birds approaching slaughter age. This strategy enabled the restriction periods to be reduced (i.e. if infected young turkeys, breeders or layers were kept on the farms, the restriction period could be several months) and hence facilitated faster restocking.
Restriction measures on the movement of live poultry, vehicles and staff must also be imposed in the areas at risk.
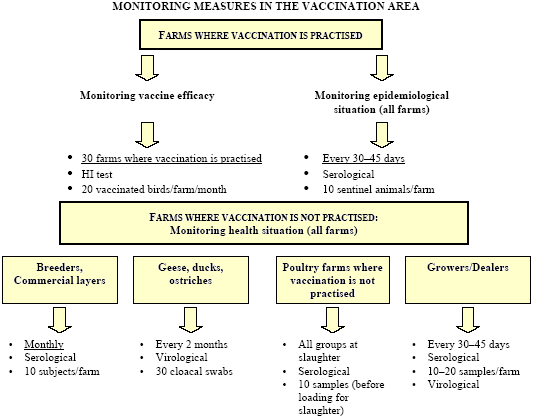
Finally, if vaccination is the proposed strategy, vaccine banks should be available for immediate use and a contingency plan must be enforced. A territorial strategy must also be implemented. It must include restriction measures (Tables 2 and 3) and an ongoing set of adequate controls (Table 4) that enable public authorities to establish whether or not the virus is circulating in the vaccinated population and to assess the efficacy of the vaccination programme.
6. Applications in the field
Inactivated homologous vaccines
Inactivated homologous vaccines have recently been used in the attempt to control AI infections in Pakistan and in Mexico (22), but in these specific conditions they have not have been successful in eradicating the infection. Conversely, the use of this vaccination strategy was successful in one instance in Utah, United States of America (USA) (13). The reason for the discrepancy between the results may lie in the efficacy of direct control measures, which must be implemented to support a vaccination campaign.
Inactivated heterologous vaccines
A vaccination strategy using inactivated heterologous vaccines has been used successfully for many years in Minnesota, USA (15), however in these instances vaccination was never implemented to control infections caused by viruses of the H5 or H7 subtypes. In addition the heterologous neuraminidase was not used as a marker of infection.
In Italy during 2000–2002, this vaccination strategy was used to supplement control measures for the eradication of the H7N1 LPAI virus (9). To control the re-emergence of LPAI virus and to develop a novel control strategy, a coordinated set of measures, including strict biosecurity, a serological monitoring programme and a ‘DIVA’ strategy were enforced (Commission Decision 2001/721/CE as amended; ref. 10).
The ‘DIVA’ strategy was based on the use of an inactivated oil emulsion heterologous vaccine containing the same H subtype as the field virus, but a different N, in this case an H7N3 strain. The possibility of using the diverse N group to differentiate between vaccinated and naturally infected birds was achieved through the development of an ‘ad hoc’ serological test to detect the specific anti-N1 antibodies (6).
Control of the field situation was achieved through an intensive sero-surveillance programme aimed at the detection of the LPAI virus through the regular testing of sentinel birds in vaccinated flocks and through the application of the anti-N1 antibody detection test. Serological monitoring was also enforced in unvaccinated flocks, located both inside and outside the vaccination area. In addition, the efficacy of the vaccination schemes was evaluated in the field through regular serological testing of selected flocks.
After the first year of vaccination, the epidemiological data collected indicated that the H7N1 virus was not circulating. This was considered sufficient by the EU Commission to lift the marketing restrictions on fresh meat from vaccinated poultry provided that animals had been tested using the discriminatory test with negative results (Commission Decision 2001/847/CE; ref. 10).
It is clear that due to the unpredictable nature of the epidemiology of this disease, which could result in the introduction of other AI subtypes, this solution is to be considered ‘tailored’ for a given epizootic.
| Table 2. Basic restriction and monitoring measures to be enforced on the movements of live poultry and poultry products originating from and/or destined for farms or plants located in the vaccination area (VA) | ||||
| Commodity | Restrictions on movements to the VA | Restrictions on movements inside the VA | Restrictions on movements out of the VA | |
| Hatching eggs | shall be transported directly to the hatchery of destination (and their packaging) must be disinfected before dispatch tracing-back of egg lots in the hatchery shall be guaranteed | must originate from a vaccinated or unvaccinated breeding flock that has been tested, with negative results, according to Table 4 shall be transported directly to the hatchery of destination (and their packaging) must be disinfected before dispatch tracing-back of egg lots in the hatchery shall be guaranteed | must originate from a vaccinated or unvaccinated breeding flock that has been tested, with negative results, according to Table 4 shall be transported directly to the hatchery of destination (and their packaging) must be disinfected before dispatch tracing-back of egg lots in the hatchery shall be guaranteed | |
| Day-old chicks | must be destined for a poultry-house where: no poultry is kept cleansing and disinfection operations have been carried out | must originate from hatching eggs satisfying the conditions mentioned above must be destined for a poultry-house where no poultry is kept and where cleansing and disinfection operations have been carried out | must originate from hatching eggs satisfying the conditions mentioned above must be destined for a poultry-house where no poultry is kept and where cleansing and disinfection operations have been carried out | |
| Ready-to-lay pullets | must be: housed in a poultry-house where no poultry has been kept for at least 3 weeks, and cleansing/disinfection operations have been carried out vaccinated at the farm of destination | must: have been regularly vaccinated against AI have been tested, with negative results, according to Table 4 be destined for a farm located in the VA and housed in a poultry-house where no poultry has been kept for at least 3 weeks, and cleansing/ disinfection operations have been carried out be officially inspected within 24 hours before loading be virologically and serologically tested with negative results before loading (sentinel birds) | must: not have been vaccinated have been tested, with negative results, according to Table 4 be destined for a poultry-house where no poultry has been kept for at least 3 weeks, and cleansing/disinfection operations have been carried out be officially inspected within 24 hours before loading be virologically and serologically tested with negative results before loading | |
| Poultry for slaughter | must be sent directly to the abattoir for immediate slaughter must be transported by lorries that operate, on the same day, only on farms located outside the VA lorries must be washed and disinfected under official control before and after each transport | shall undergo a clinical inspection within 48 hours before loading must be directly sent to the abattoir for immediate slaughter must be serologically tested before loading the abattoir must guarantee that accurate washing and disinfection operations are carried out under official supervision shall be transported by lorries that operate, on the same day, only on farms located inside the VA lorries must be washed and disinfected before and after each transport | shall undergo a clinical inspection within 48 hours before loading must be sent directly to an abattoir designated by the competent veterinary authority for immediate slaughter must be serologically tested before loading the abattoir must guarantee that accurate washing and disinfection operations are carried out under official supervision shall be transported by lorries that operate, on the same day, only on farms located inside the VA lorries must be washed and disinfected before and after each transport | |
| Table eggs | must be: sent directly to a packaging centre or a thermal-treatment plant designated by the competent authority transported using disposable packaging materials that can be effectively washed and disinfected | must: originate from a flock that has been tested, with negative results, as laid down in Table 4 be sent directly to a packaging centre or a thermal-treatment plant designated by the competent authority be transported using disposable packaging material or packaging material that can be effectively washed and disinfected | must: originate from a flock that has been tested, with negative results, as laid down in Table 4 be sent directly to a packaging centre or a thermal-treatment plant designated by the competent authorities be transported using disposable packaging material or packaging material that can be effectively washed and disinfected | |
| Table 3. Basic restrictions to be applied to the trade of fresh meat produced from poultry originating from the vaccination area (VA) | ||
| Commodity | Unrestricted to international trade | Restricted to national trade |
| Fresh poultry meat | Originating from birds vaccinated against AI with a heterologous subtype vaccine can be dispatched to other countries, provided that the meat comes from slaughter bird flocks that:
|
Originating from holdings located in the VA cannot be dispatched to other countries, if produced from poultry:
|
Recombinant vaccines
The only field experience with recombinant vaccines has been in Mexico, where they have been used in the vaccination campaign against the H5N2 virus. AI has not been eradicated in Mexico, probably because an eradication programme based on a territorial strategy and including monitoring and restriction was not established.
Recombinant live vectored vaccines also enable the differentiation between infected and vaccinated birds as they do not induce the production of antibodies against the nucleoprotein antigen, which is common to all AI viruses. Therefore, only field-infected birds will exhibit antibodies to the agar gel precipitation test or enzyme-linked immunosorbent directed towards the detection of group A (nucleoprotein) antibodies.
As these vaccines have encountered some difficulties in licensing, their use is restricted to countries in which they are legally available. In addition, these vaccines will not induce replication and protective immunity in birds that have had field exposure to the vector (i.e. fowlpox or infectious laryngotracheitis viruses) (16, 19). As serological positivity to these viruses is widespread (due to field exposure and vaccination) in the poultry population and can in some instances be unpredictable, the use of these vaccines is limited to a population that is seronegative to the vector virus. The use of these vaccines is also restricted to species in which the vector virus will replicate. For example, ILTV will not replicate in turkeys, and as these birds are particularly important in the epidemiology of AI, the use of this vaccine is limited to areas in which turkeys are not present.
7. Discussion
From the data presented, it appears that emergency vaccination is a sensible option if there is evidence of the introduction of a highly transmissible AI virus into a densely populated poultry area, or whenever the epidemiological situation indicates that there could be massive and rapid spread of infection. Emergency vaccination should also be considered when birds of high economic value (e.g. pedigree flocks) or rare (endangered) birds are at risk of infection. It is clear that vaccination represents a tool to aid eradication, and will be a successful tool only if coupled with movement restrictions and increased biosecurity.
Considering the advantages and disadvantages of the products and diagnostic tools that are available currently, if no recombinant products are licensed in a country, heterologous vaccination rather than homologous vaccination should be practised in emergency situations. The main reason for this is that it would enable the differentiation of vaccinated from naturally exposed birds through the development/application of an appropriate test. At present only the anti-neuraminidase based test has been validated and is available. In our opinion however, this test represents a starting point on which future developments of the ‘DIVA’ strategy can be based. The development of novel candidate vaccines and of additional tests that enable the detection of field infection in vaccinated populations should be a priority for the pharmaceutical industry and for research institutions because, for all the reasons listed above, vaccination is already an option for the control of AI.
If a country has access to licensed recombinant products, the use of these vaccines is acceptable taking into consideration the immune status of the population against the vector because seropositivity impedes the replication of the vector virus and therefore the establishment of immunity. The issue of the replicating capacity of the vector in different species must also be addressed.
In conclusion, recent events including devastating epizootics in densely populated poultry areas, public health concern on animal welfare issues and the introduction of novel technology to vaccinology have encouraged consideration of alternative control strategies for OIE List A diseases that were unthinkable only a few years ago. This has also been supported by the development of reliable, sensitive and specific diagnostic companion tests. Countries, areas and enterprises at risk of infection should imperatively implement surveillance programmes and have contingency plans in case of a disease outbreak, which may include vaccination. If the latter is considered as an option, the contingency plan must, among other issues, foresee the establishment of licensed vaccine banks that enable the ‘DIVA’ strategy to be enforced thus safeguarding animal health, animal welfare and international trade.
Source: Office International des Epizooties - January 2004