

Aris Malo, DVM,* clears the air about IB variants, the challenges of control programs and the value of using a Protectotype strategy with vaccines of different serotypes.
Q:How big of a problem is infectious bronchitis (IB)?
AM: IB remains a highly infectious, common and costly disease in commercial poultry around the world. The virus can spread through an entire flock in only 1 or 2 days, causing widespread morbidity and variable mortality.
Q: What are the common manifestations of the IB virus?
AM: The disease starts in the respiratory tract and can cause respiratory signs such as gasping, coughing and nasal discharge, but the virus can also spread quickly throughout the bird, affecting the reproductive tract and kidneys.
Q:What are the economic losses that occur due to IB?
AM: In broilers, producer losses
from IB occur due to poor
growth and feed conversion,
secondary bacterial infections
that require antibiotic treatment
and increased condemnations
at slaughter.
In layers and breeders, egg
production and quality are adversely affected. Losses from
kidney damage can occur in
broilers, layers and breeders.
Q: Why is IB so difficult to control?
AM: New IB serotypes are
emerging all the time. The
genetic makeup of the IB virus
can be altered by spontaneous
mutation or by recombination,
which is the exchange of
genetic information with
another genome.
Many of the IB virus-variant
strains that develop don’t
survive long enough to become
a problem, but some do, and
those are the ones we have to
worry about — the surviving,
pathogenic variants.
Q:What new variants are the most important?
AM: One is the variant known as IB 4/91, which is found in many parts of the world, including Europe. Another is called QX — it’s thought that this variant originated in China, then spread to Europe. It can lead to proventriculitis, severe kidney damage, permanent damage in the oviduct (resulting in so-called “false-layers”) and poor egg production.
Q: There’s been much discussion lately about developing a “Protectotype” protocol. What do scientists mean by that? And how does it relate to IB-management programs?
AM: Some IB virus serotypes are
able to cross-protect against
other IB serotypes; these have
become known as Protectotypes.
There’s a reason for this. New IB
variants can arise due to very
small changes in the makeup of
the IB virus, but the rest of the
virus’ genetic makeup remains
the same. This is why some
cross-protection is thought
to occur.
An example of a Protectotype
protocol is one featuring the
live vaccines Nobilis IB Ma5
and Nobilis IB 4/91. The Ma5
vaccine is based on the classical
Massachusetts IB virus serotype,
while the 4/91 vaccine is based
on the 4/91 IB variant serotype.
Used together, they can provide
broad protection against an
IB challenge.
Q: What evidence is there that the Protectotype protocol works?
|
“Many of the IB virus-variant strains that develop don’t survive long enough to become a problem, but some do, and those are the ones we have to worry about — the surviving, pathogenic variants.” |
ArIs Malo, DVM |
In one study, specific-pathogenfree chickens received Nobilis IB Ma5 at 1 day of age; it provided excellent protection against the USA Arkansas IB isolate and IB isolates from Brazil and Japan. Nobilis IB 4/91 used alone at 14 days of age protected against the same three isolates plus an IB isolate from Italy. However, the best cross-protection occurred when both vaccines were used and when Ma5 was administered before, rather than at the same time or following, the 4/91 vaccine.1
In 2008, Italian investigators concluded from their study that the use of these two vaccines may be “instrumental in reducing the economic impact of QX IB virus infections” on layer and broiler farms.2
Another study demonstrated that the 4/91 live strain used alone, and especially live Ma5 plus 4/91, protected well against a nephropathogenic IB virus (B1648) — a strain that attacks the kidneys.3
Q:What about field experience with the Protectotype protocol?
AM: The results from studies are
being proven true in the field.
A recent case occurred on two
Middle Eastern broiler farms.
One had been using only the
Ma5 vaccine and the other was
using H120 vaccine.
After the Protectotype protocol
utilizing Nobilis IB Ma5 and
Nobilis IB 4/91 was initiated,
mortality on both farms declined
and bodyweight improved.
More specifically, at one farm
following just two cycles of the
Protectotype protocol, mortality
dropped from 35% to less than
8%. Furthermore, bodyweight
increased from 1.406 grams to
1.600 grams — nearly 14% —
and days until slaughter decreased
from 36.05 to 34 days.
Q:Why not develop new vaccines for new strains that emerge?
AM: Developing a new vaccine for
every significant IB variant that
emerges would be impractical
and costly, especially in today’s
regulatory environment. A recent
survey conducted in the UK
demonstrated that a wide variety
of IB strains is circulating, and the
situation is likely to be similar in
other European countries.
Because existing vaccines can
effectively control many IB
strains, it makes more sense to
use what’s already available.
Q:Could an IB H120 vaccine be used instead of an Ma5-strain vaccine as part of the Protectotype protocol?
AM: In some circumstances, an
IB H120-strain vaccine may be
adequate, but generally, field
results have been better when
the Ma5-strain vaccine is used.
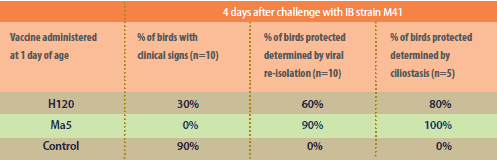
Detailed genetic studies
conducted in Brazil have demonstrated
that even though H120
and Ma5 are both from the
Massachusetts IB serotype, they
differ. The Nobilis IB Ma5 strain’s
S1 subunit of the spike gene was
found to be structurally different
compared to an H120 strain.
It’s thought that this finding
explains why the Nobilis IB Ma5
strain appears to be more
immunogenic than H120.
As far back as the 1980s, a
study conducted by
Intervet/Schering-Plough
Animal Health indicated that
Ma5 may be more immunogenic
than H120.4
Q:What if I also need to vaccinate against Newcastle disease?
AM: Nobilis IB Ma5 will not interfere with the Nobilis Clone 30 Newcastle disease vaccine. If other live-IB vaccines are used, they should be given 1 week apart from additional respiratory disease vaccines to prevent interference, which occurs when both vaccines compete for the same receptor sites in the trachea.
Q:Won’t biosecurity and good husbandry practices keep IB under control?
AM: Biosecurity and good husbandry practices are crucial for control of IB but are seldom sufficient. They must be used along with IB vaccines — which must be administered properly to get the best results.
References1 Cook, J.K.a., et al. Breadth of protection of the respiratory tract provided by different live-attenuated infectious bronchitis vaccines against challenge with infectious bronchitis viruses of heterologous serotypes. Avian Pathology 1999:28;477-485.
2 Terregino, C., et al. Pathogenicity of a QX strain of infectious bronchitis virus in specific-pathogen-free and commercial broiler chickens, and evaluation of protection induced by a vaccination programme based on the Ma5 and 4/91 serotypes. Avian Pathology 2008:37; 487-493.
3 Cook, J.K.a., et al. Protection of chickens against renal damage caused by a nephropathogenic infectious bronchitis virus. Avian Pathology 2001:30;423-426.
4 Torres, C.a., universidade de são Paulo, Brazil, et al. on the molecular basis of the higher protection by nobilis IB Ma5 strain of IBV against infectious bronchitis when compared to H120. article on file at Intervet/schering-Plough animal Health.
More Issues









