
Getting a grip
Why are researchers investigating the role of alpha-toxin in
the development of necrotic enteritis? Two scientists share
insights from studies that could impact the management of
NE, a serious and costly disease.
Strong evidence that alpha-toxin plays
a role in the development of necrotic
enteritis (NE) has been demonstrated
in studies utilizing an alpha-toxin test
kit and immunohistochemistry,
Dr. Joan Schrader said at the World’s
Poultry Congress.
Alpha-toxin is a toxic protein secreted by
the bacterium Clostridium perfringens. It
is also a component of C. perfringens type
A toxoid, a conditionally licensed US
vaccine that is administered to breeders
for control of NE in progeny chicks.
The vaccine was developed by Intervet/
Schering-Plough Animal Health, said
Schrader, a scientist with the company.
The recent availability of a commercial
diagnostic test-strip kit designed to detect
C. perfringens and alpha-toxin in feces provided a new way to evaluate the role
of alpha-toxin in the development of
NE, she said. Schrader also conducted
immunohistochemistry to physically
demonstrate alpha-toxin at the lesion site.
The test utilizes monoclonal antibodies to
both C. perfringens type A and alpha-toxin
bound to a paper strip. When the strip is
exposed to these antigens in solubilized
chicken feces, one line develops color in
the presence of C. perfringens type A,
and a second line develops color in the
presence of alpha-toxin.
Study details and results
For the study, 52 commercial, day-old
broiler chicks were placed in floor pens at
the company’s R&D facility in Elkhorn,
Nebraska. Thirty-five test chicks were
housed in one hut, and the remaining chicks were housed in another hut and
were used as controls.
Chicks were fed a non-medicated starter
ration for the first 5 days and were then
switched to a high-protein diet for the
remainder of the study. When the test
chicks were 19, 20 and 21 days of age,
a C. perfringens type A challenge was
performed by oral gavage.
At 23 days of age, fecal material was
collected from the caudal rectum/cloaca
of each chicken and tested according to
the kit instructions. Three strips were
tested for each sample, Schrader said.
Chickens were also scored for NE lesions,
which were used to determine the true
prevalence of NE, and the ability of the
test strips for detecting C. perfringens and
alpha-toxin was determined, she said.
The overall prevalence of positive test,
according to lesion score, for C. perfringens
in birds was 33% for score 0 (6/18), 18%
for score 1 (14/78), 19% for score 2
(9/48), 61% for score 3 (11/18) and 88%
for score 4 (16/18). The incidence of
positive test strips for C. perfringens was
not different between birds positive or
negative for NE (Table 1), Schrader said.
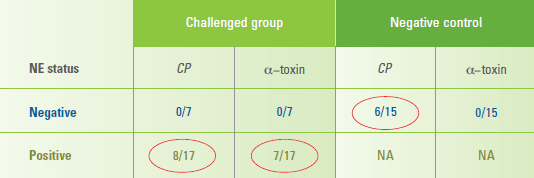 Table 1.
Table 1. Incidence of positive test strips for C. perfringens was not different between birds positive
or negative for NE.
CP = Clostridium perfringens
Note: Alpha-toxin was only detected in chickens positive for NE
Alpha-toxin was not detected by the test
strips until lesion scores reached 3 or 4:
The test kit was able to detect alpha-toxin
in 37% (7/18) of tests among chickens
with lesion scores of 3 and in 71% (13/18)
of tests among chickens with lesion scores
of 4, she said.
The study showed a good correlation
between lesion score and the detection
of alpha-toxin, with higher lesion scores
resulting in greater detection of alphatoxin
with the test kit, Schrader said.
In addition, the finding that high lesion
scores correlated with positive test-strip
results for C. perfringens and alpha-toxin
at the site of NE lesions “supports the
hypothesis that the severity of the gross lesions is directly proportional to the
number of C. perfringens present and
amount of alpha-toxin produced,”
she said.
Immunohistochemistry results
Schrader then performed immunohistochemistry
on NE lesions, a technique
that has been widely used to detect the
presence of disease agents in tissues.
To perform the test, a very thin-sliced
tissue sample is fixed to a slide. An
“anti-antibody” that has fluorescent or
pigmented material is added to the slide
and binds to the antibody in question if
that antibody is present. In this case,
“There was clearly a positive binding of
antibodies,” Schrader explained (see
Figure 1).
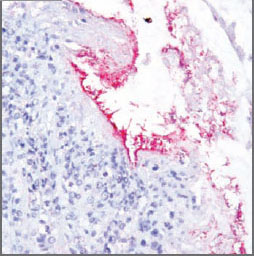 Figure 1.
Figure 1. An “anti-antibody” with fluorescent
or pigmented material binds to the antibody
in question if that antibody is present.
The results of the study, Schrader
concluded, “strongly demonstrate the
involvement of alpha-toxin in the disease
of necrotic enteritis.”
Although the test kit was useful for the
purposes of her study, she said, it would
not be particularly helpful in the field
for producers trying to detect subtle,
subclinical NE that can go unnoticed but
eat away at performance. It would pick up
birds with overt, clinical NE and high
lesion scores, and “by then you’d already
know the birds are sick.”
US study suggests
alpha-toxin plays role
in cause of NE
Vaccination of broilers with recombinant alpha-toxin
protected broilers against an experimental challenge with
Clostridium perfringens, suggesting that alpha-toxin plays a
role in the pathogenesis of necrotic enteritis (NE).
In the study, broiler chicks were vaccinated subcutaneously with
recombinant alpha-toxin at 5 and 15 days of age, then 10 days
later were challenged with C. perfringens, the cause of necrotic
enteritis. The challenge was administered twice daily for 4
consecutive days by mixing C. perfringens cultures with feed.
Non-vaccinated birds challenged with C. perfringens developed
NE at the rate of 87.8%, but only 54.9% of vaccinated birds
developed lesions. In addition, non-vaccinated birds had lesion
scores averaging 2.37, compared to 1.35 in vaccinated birds, write
K. K. Cooper and colleagues at the University of Arizona, Tucson,
in the June 2008 issue of Veterinary Microbiology.
Vaccination also produced an antibody response — post-vaccination
anti-alpha-toxin titers in vaccinated birds were more than 5-fold
greater than in non-vaccinated birds. After challenge, vaccinated
birds had average IgG (IgY) titers >15-fold higher compared to those
of non-vaccinated birds, the investigators say.
NE in poultry has re-emerged as a concern for poultry producers due
in part to the ban on in-feed antimicrobial growth promoters, but the
results of this study, say the investigators, suggest that alpha-toxin
may serve as an effective immunogen and, as such, may play a role
in pathogenesis of necrotic enteritis.
PRESCOTT: PROTEIN PROVIDES
EXCELLENT
PROTECTION
AGAINST NECROTIC
ENTERITIS
Independent research by Canadian
investigators using state-of-the-art
technology confirms that alpha-toxin,
a secreted protein from Clostridium
perfringens, plays a key role in the
development of necrotic enteritis (NE)
in broilers and that other proteins may
also be involved in the pathogenesis of
this complex disease.
Immunization with alpha-toxin provided
almost total protection of broiler chickens
against NE, while other secreted proteins
produced by virulent C. perfringens yielded
various degrees of immunity, Dr. John F.
Prescott, of the University of Guelph,
Ontario, said at a meeting of the Ontario
Association of Poultry Practitioners held
in 2008 in Guelph.
Necrotic enteritis has become an economically
important disease for the broiler
industry. Although the cause is known
to be C. perfringens, exactly how this
bacterium causes NE has been uncertain,
Prescott explained.
The major culprit has been assumed to be
alpha-toxin. Earlier this year, however,
Australian researchers published an article
about a novel C. perfringens toxin they identified, called NetB. In widely publicized
findings, they indicated that NetB
was the main cause of NE and, on the
basis of their carefully conducted research,
discounted the role of alpha-toxin.
Canada study
Studies by Prescott and his colleagues,
however, show that immunization with
alpha-toxin provided the best protection
against a severe C. perfringens challenge
with a virulent strain that contains the
NetB gene.
In their studies, which Prescott
reviewed at the Ontario meeting, several
proteins secreted by C. perfringens were
evaluated for their ability to protect
broilers against the virulent strain of the
organism. The proteins were alpha-toxin,
glyceraldehyde-3-phosphate dehydrogenase,
pyruvate:ferredoxin oxidoreductase
(PFOR), fructose 1,6-biphosphate
aldolase, and a fifth one called hypothetical
protein (HP).
Broilers were immunized two to three
times with one of the proteins, then one
week after their last immunization, they
were challenged with the virulent strain, which was administered in feed at 4 weeks
of age.
The severity of the challenges differed; a
mild challenge, for instance, involved
feeding the virulent strain to birds three
times daily for 3 days, and the most severe
challenge involved feeding the virulent
strain daily to birds continuously for 5
days. The severity of each challenge was
confirmed by NE lesion scores in nonimmunized
but challenged control birds.
All the proteins significantly protected
broilers against the relatively mild
challenge. For the more severe challenge,
alpha-toxin, PFOR and HP provided
significant protection, Prescott said.
Alpha-toxin provided
best protection
The greatest protection against severe
challenge, however, occurred in birds that
were primed twice with alpha-toxoid —
a toxin that is altered so it is no longer
toxic but still initiates immunity —
and then boosted with active, purified
toxin, Prescott and colleagues found in their study, published in 2007 in the
September issue of Clinical and
Vaccine Immunology.
In addition, serum and intestinal washings
from protected birds had high antigenspecific
antibody titers for all proteins
used in their study, the researchers found.
NetB may be marker
Prescott and associates also used polymerase
chain reaction to test the virulent
challenge strain and found it was positive
for the NetB gene.
“The fact...that immunization with
alpha-toxin strongly protected birds
against experimental NE caused by a
NetB-containing isolate suggests that
alpha-toxin actually is critical to the
development of NE, and perhaps that
NetB may only initiate infection,”
he said.
“I know that the Australian workers think
that the success of antibody against alphatoxin
in protecting so well against NE is
because it may interfere with the secretion
of all proteins by this organism, including,
for example, the secretion of NetB. It will be hard to prove this, and actually may
not matter if alpha-toxin immunization
works so well,” he added.
In addition, unpublished observations
from Ontario show that genetically
unrelated isolates from sick birds in
flocks with NE “were systematically
NetB-positive, whereas isolates from
healthy birds at slaughter were usually
negative” for NetB, he said.
“Almost but not quite all [C. perfringens]
isolates from birds with NE or from flocks
experiencing NE have NetB, so it’s a good
marker for a strain of C. perfringens that
causes necrotic enteritis,” Prescott said.
Asked by Intestinal Health why the search
continues for other secreted proteins when
it has already been shown that alpha-toxin
can protect broilers from NE, Prescott
said, “It will help us understand NE
better, though I agree that alpha-toxin
should be the main focus. On the basis
of the findings of protection of birds
following immunization, alpha-toxin
apparently has a central role in NE,” but
there may be an advantage to using
more than one protein.
Proteins differ, he added, in their
structure, in their activity, including
toxicity, and in their targets.
Favors vaccine
Methods for controlling NE might
include probiotics to provide bacterial
competition for C. perfringens or killing
C. perfringens with novel antibiotics,
but Prescott favors immunization.
“I think a vaccine probably has the
most promise because it should be the
most reliable. I like the idea of an oral
vaccine because it could also be used to
deliver other antigens and products,” said
Prescott, who has been experimenting
with an orally administered, attenuated
salmonella vaccine vector with
C. perfringens antigens.
Even though there is still much to be
learned about NE, he predicts rapid
advancement in the quest to conquer the
disease, thanks to large-scale genome
sequencing and other technologies. Due
to these advances, “scientists working on
NE around the world have made more
strides in the last 3 to 4 years than in the
previous 25 years,” he said.
NE is a
complicated
disease
Finding ways to prevent or control necrotic enteritis (
NE) in
broilers is challenging because Clostridium perfringens, the
bacterium that causes the disease, has chameleon-like qualities,
and other factors, such as management, may be involved.
At the World’s Poultry Conference this summer in Brisbane,
Dr. John Prescott, of the University of Guelph, called
C. perfringens “an absolute thug.”
The bacterium is “exquisitely adapted as an environmental
anaerobe to grow very rapidly in injured or dead animal tissue.
Consider that Escherichia coli doubles every 20 minutes. In
contrast, C. perfringens is the fastest growing organism known
and, under optimal conditions, doubles every 8 to 10 minutes,”
he said.
“It is superbly designed to take advantage of injured tissue,” he
said. It secretes multiple toxins and enzymes that maximize the
destruction of tissues.
Dr. Joan Schrader, a scientist with Intervet/Schering-Plough
Animal Health who has researched NE and helped develop the
company’s Clostridium perfringens type A toxoid for broilers,
agrees.
“It’s as though virulent C. perfringens has an arsenal of toxins it
can produce, and depending on the environment the bacterium
is in, it will use the toxins that are most advantageous for the
circumstances. It’s very much a multifactorial disease,” she says.
Schrader echoes Prescott’s opinion, saying that while “alphatoxin
is a key player, other secreted proteins from C. perfringens
may be involved in development of this complicated disease.”
In addition, secreted proteins may be only part of the story.
In his OAPP talk, Prescott pointed to published evidence that
dietary components might adversely affect intestinal motility or
damage intestinal mucosa, which in turn affect C. perfringens
toxin production or the growth of C. perfringens. Coccidial
infection can be a contributing factor too, he said.
“The interaction of [C. perfringens] with other intestinal microflora,
including non-NE isolates, and the effect of other microflora
on intestinal innate immunity” may be important, he said.
There’s no question, he and Schrader say, that NE is a complex
infection.
Alpha-toxin gene linked to necrotic enteritis in India
A study conducted on broilers
from India confirmed that Clostridium
perfringens type A was the cause of
necrotic enteritis (NE) and that alpha-toxin
may play a significant role in development
of the disease, say Arunava Das of the
Bannari Amman Institute of Technology,
and associates.
After six broilers died at 2 to 3 weeks of
age on a poultry farm in Meghalaya, India,
investigators performed scanning electron
microscopy (SEM) and evaluated intestinal
contents and liver samples.
SEM revealed massive necrosis and
complete destruction of the intestinal villi
within the intestinal mucosa. Bacterial
isolation confirmed that C. perfringens
was the cause. Polymerase chain reaction
(PCR) testing of 10 clinical isolates
showed they all harbored the alpha-toxin gene of C. perfringens; four were positive
for the beta2 toxin gene; and none were
positive for the beta, epsilon, iota or
enterotoxin genes.
All isolates derived from NE belonged to
C. perfringens type A and there was
97.6% to 100% homology among the
C. perfringens isolates, they write in a
recent issue of the International Journal
of Poultry Science (7 (6): 601-609, 2008).
The study confirms that C. perfringens
type A is the most predominant one
associated with necrotic enteritis in
broiler chickens in this region of India
and that the alpha-toxin gene might play
a significant role in the pathogenesis
of the disease in broiler chickens, the
investigators conclude.
Back to North American Edition (#2)











